YolTech Therapeutics Initiates Clinical Trial with First Dose of YOLT-203 for In Vivo Gene Editing in PH1 Therapy
YolTech Therapeutics, a trailblazing company in the clinical-stage gene editing arena, has reached a significant milestone: the first patient has been administered YOLT-203, their cutting-edge in vivo genome editing candidate. This event marks the beginning of an Investigator-Initiated Trial (IIT) and is a groundbreaking moment for the use of in vivo gene editing therapies in treating Primary Hyperoxaluria Type 1 (PH1). YOLT-203 is formulated as a potentially curative, single-dose therapy, leveraging YolTech’s proprietary YolCas12™ editor. This trial elevates the standards in the field, expanding the possibilities of gene editing in the treatment of severe genetic disorders.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Primary hyperoxaluria is an inherited ailment characterized by an excessive production of oxalate in the liver, resulting in elevated levels of oxalate excreted by the kidneys. Among its subtypes, PH1 is the most frequently diagnosed and is an autosomal recessive disease that begins in childhood. This form of the disorder arises due to mutations in the AGXT gene and a lack or malfunction of the enzyme alanine-glyoxylate aminotransferase. Eventually, most individuals suffering from PH1 progress to kidney failure, necessitating rigorous hemodialysis as a temporary measure before undergoing combined liver and kidney transplantation to correct the metabolic flaw in the liver and replace severely damaged kidneys.
YOLT-203, created independently by YolTech, is built upon the company’s unique YolCas12™ editor, a cutting-edge CRISPR/Cas gene editing technology developed via YolTech’s metagenomics and protein engineering platform, HEPDONE® (High-Throughput Evolution Platform for Discovery and Optimization of Novel Editors) system. YolCas12™ has shown remarkably high gene editing efficiency in both prokaryotic and eukaryotic cells and has been validated for high efficacy in vivo gene editing through LNP-mRNA delivery in mice and non-human primates. YOLT-203 leverages the YolCas12™ editor to target the HAO1 gene, effectively reducing pathogenic protein expression in pre-clinical studies, and holds promise as a viable treatment for both child and adult patients with PH1.
The clinical investigation of YOLT-203, spearheaded by researchers, follows a single-arm, open-label, dose-escalation design to assess the safety and tolerability of YOLT-203 among PH1 patients in China. The trial plans to enroll 7 participants. The initial adult participant received the dose on August 5, 2024, and the first pediatric patient was treated on August 20, 2024. This trial marks a groundbreaking milestone as the inaugural global clinical trial of an in vivo gene editing therapy specifically targeting PH1.
“PH1 is a severe condition that can result in critical kidney damage and widespread health issues,” remarked Dr. Yuxuan Wu, Founder and CEO of YolTech. “YOLT-203, our first in vivo gene editing solution for PH1, utilizing our proprietary YolCas12™ editor, has the potential to revolutionize treatment approaches for patients enduring this challenging disease. We anticipate YOLT-203 will deliver substantial clinical improvements for both pediatric and adult PH1 sufferers, and we are eager to share further data from this initiative in the near future.”
YolTech is dedicated to advancing the clinical progress of YOLT-203 and aims to work collaboratively with regulatory bodies, medical professionals, and patient advocacy organizations to elevate the field of gene editing therapies.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
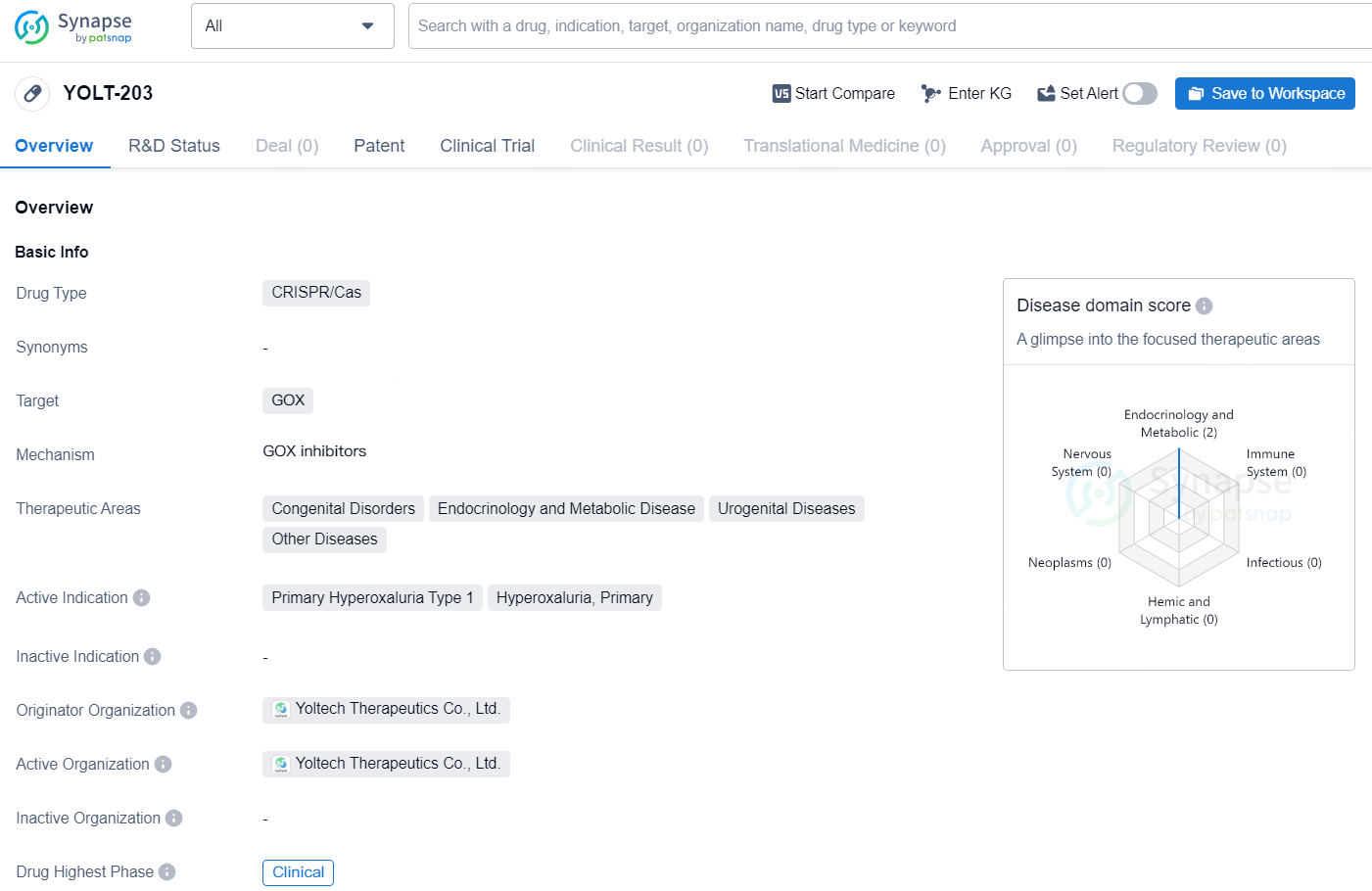
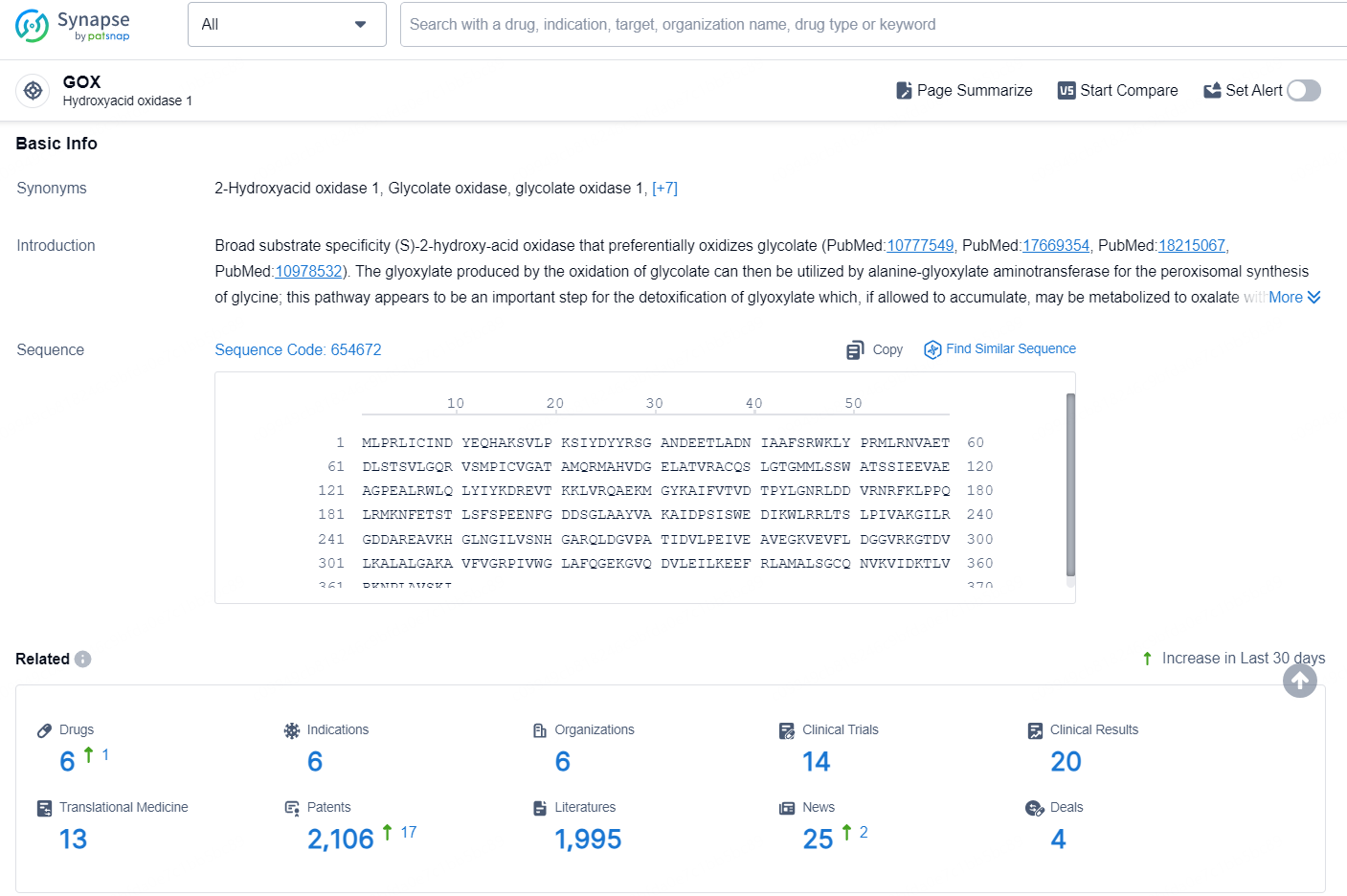
According to the data provided by the Synapse Database, As of August 27, 2024, there are 6 investigational drugs for the GOX target, including 6 indications, 6 R&D institutions involved, with related clinical trials reaching 14, and as many as 2106 patents.
YOLT-203 is a drug of CRISPR/Cas type that targets the GOX gene. It is being developed for the treatment of various therapeutic areas including Congenital Disorders, Endocrinology and Metabolic Disease, Urogenital Diseases, and Other Diseases. Its active indication includes Primary Hyperoxaluria Type 1 and Hyperoxaluria, Primary. The drug is being developed by Yoltech Therapeutics Co., Ltd., and it is currently in the highest phase of clinical trials both globally and in China.