EU Commission Greenlights KAFTRIO® Paired with Ivacaftor to treat Cystic Fibrosis in Kids Aged 2-5
Vertex Pharmaceuticals Incorporated has shared the news that the European Commission has sanctioned an extension for the label of KAFTRIO® (ivacaftor/tezacaftor/elexacaftor), to be used in conjunction with ivacaftor for the purpose of treating cystic fibrosis in children between the ages of 2 and 5 who possess a minimum of one F508del mutation in their cystic fibrosis transmembrane conductance regulator (CFTR) gene.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Clinical trial data supplemented by long-term and real-world evidence shows the significant clinical gain of KAFTRIO in eligible individuals dealing CF, noted Carmen Bozic, M.D., Executive VP, Global Medicines Development and Medical Affairs, and Chief Medical Officer, at Vertex, adding that today’s update signifies that young children across Europe can benefit from this crucial medicine.
Given CF develops in early stages of life and increasingly worsens over time, it is crucial to start treatment as soon as possible, stated Professor Marcus A. Mall, M.D., Head of Pediatric Respiratory Medicine, Immunology and Critical Care Medicine Department at Charité Universitätsmedizin Berlin. With the green light for KAFTRIO for kids as young as 2 years, these young patients can now be treated with a drug that has a potential to slow down disease progression by addressing the root cause.
Thanks to existing reimbursement schemes in Austria, Denmark, Ireland, Norway, Latvia, and Sweden, qualified patients will soon have KAFTRIO® (ivacaftor/tezacaftor/elexacaftor) in a combination regimen with ivacaftor accessible after regulatory approval by the European Commission.
In efforts to make KAFTRIO available for all suitable patients, Vertex will continue collaborating with reimbursement authorities across the European Union. Post the MHRA approval on November 15, 2023, in the U.K., due to the ongoing reimbursement agreement between Vertex and the NHS, KAFTRIO® (ivacaftor/tezacaftor/elexacaftor) in a combination with ivacaftor is accessible for kids aged 2 years and older in the U.K.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
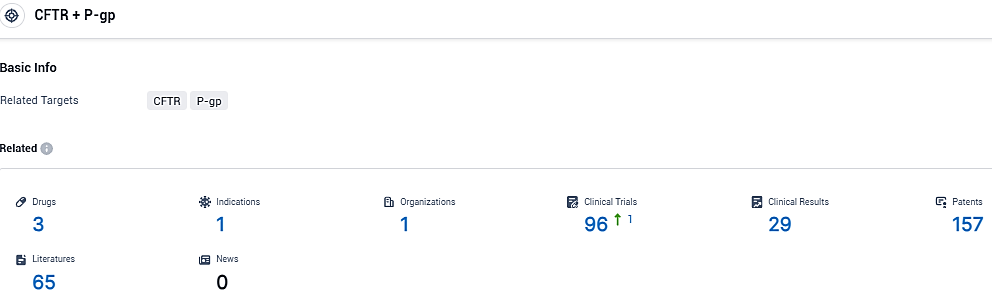
According to the data provided by the Synapse Database, As of November 30, 2023, there are 3 investigational drugs for the CFTR and P-gp target, including 1 indications, 1 R&D institutions involved, with related clinical trials reaching 96, and as many as 157 patents.
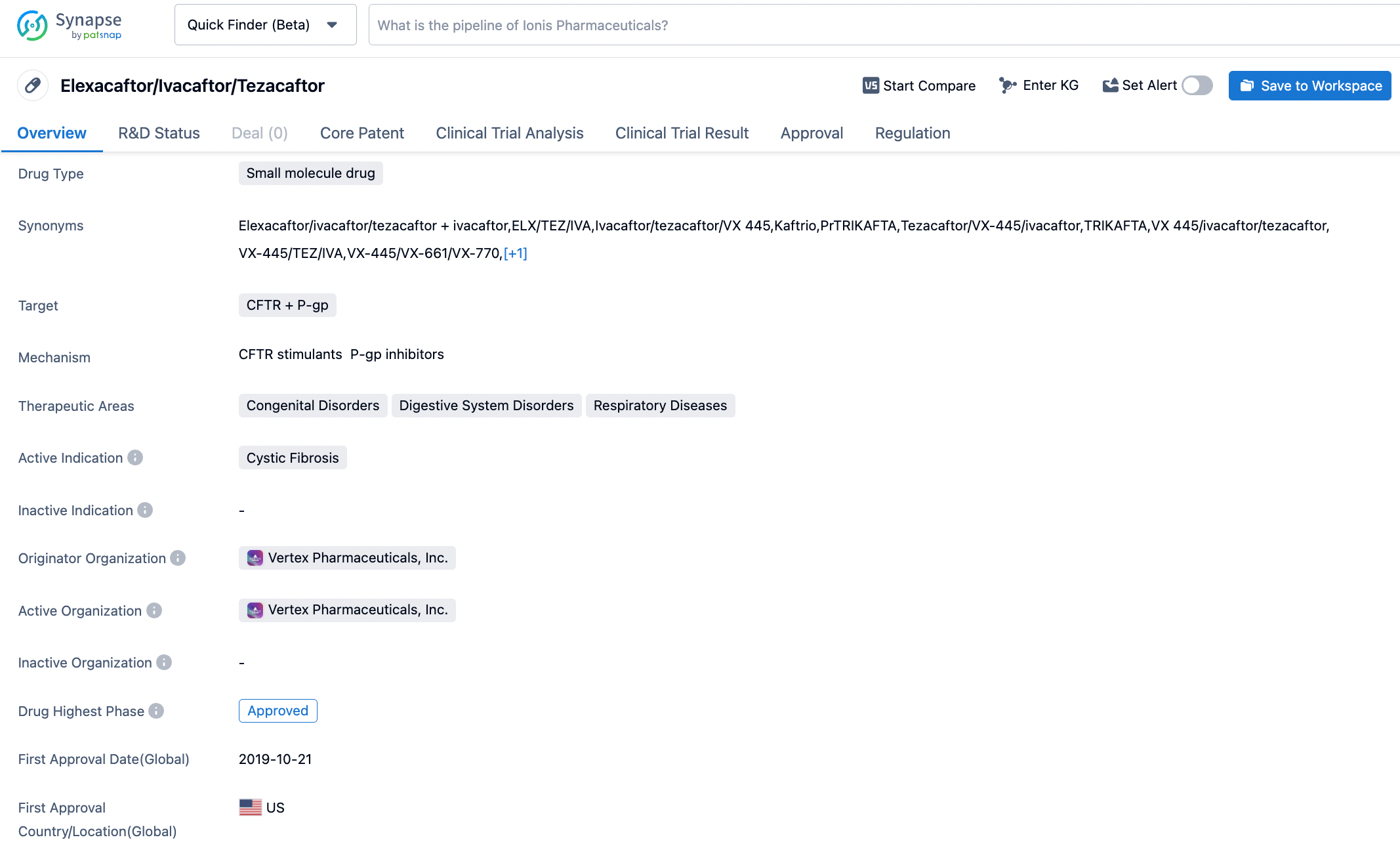
KAFTRIO® (ivacaftor/tezacaftor/elexacaftor) in combination with ivacaftor is an oral medicine designed to increase the quantity and function of the CFTR protein at the cell surface. KAFTRIO® (ivacaftor/tezacaftor/elexacaftor) in combination with ivacaftor is approved in the European Union for the treatment of cystic fibrosis in patients aged 2 years and older who have at least one copy of the F508del mutation in the CFTR gene.