Unveiling the Secrets of IL-17A Inhibitors: Stay Updated with the Latest Advances
IL-17A, or Interleukin-17A, is a cytokine that plays a crucial role in the human body's immune response. It is primarily produced by T-helper 17 (Th17) cells and other immune cells. IL-17A acts as a pro-inflammatory mediator, promoting the recruitment and activation of immune cells to sites of infection or tissue damage. It is involved in various autoimmune and inflammatory diseases, such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Understanding the role of IL-17A has led to the development of targeted therapies, including monoclonal antibodies, that specifically inhibit its activity, providing effective treatment options for patients suffering from IL-17A-mediated diseases.
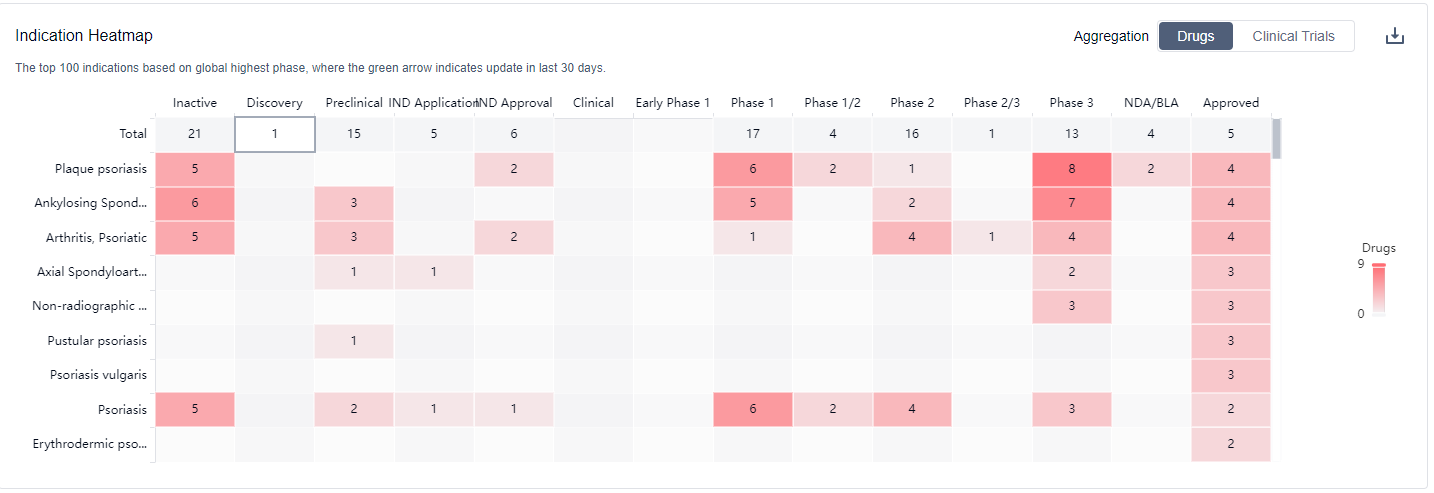
Looking ahead, IL-17A inhibitors are expected to continue to play a significant role in the treatment of various diseases. They have shown promising results in treating serious cases of psoriasis and are also used widely for the treatment of rheumatoid arthritis. However, the emergence of resistance and the lack of an effective reversal drug when bleeding occurs are challenges that need to be addressed. Ongoing research and development efforts are focused on addressing these issues and expanding the clinical applications of IL-17A inhibitors. The approval of these drugs in more countries would further contribute to addressing the unmet medical needs of individuals with immune system diseases, digestive system disorders, and skin and musculoskeletal diseases.
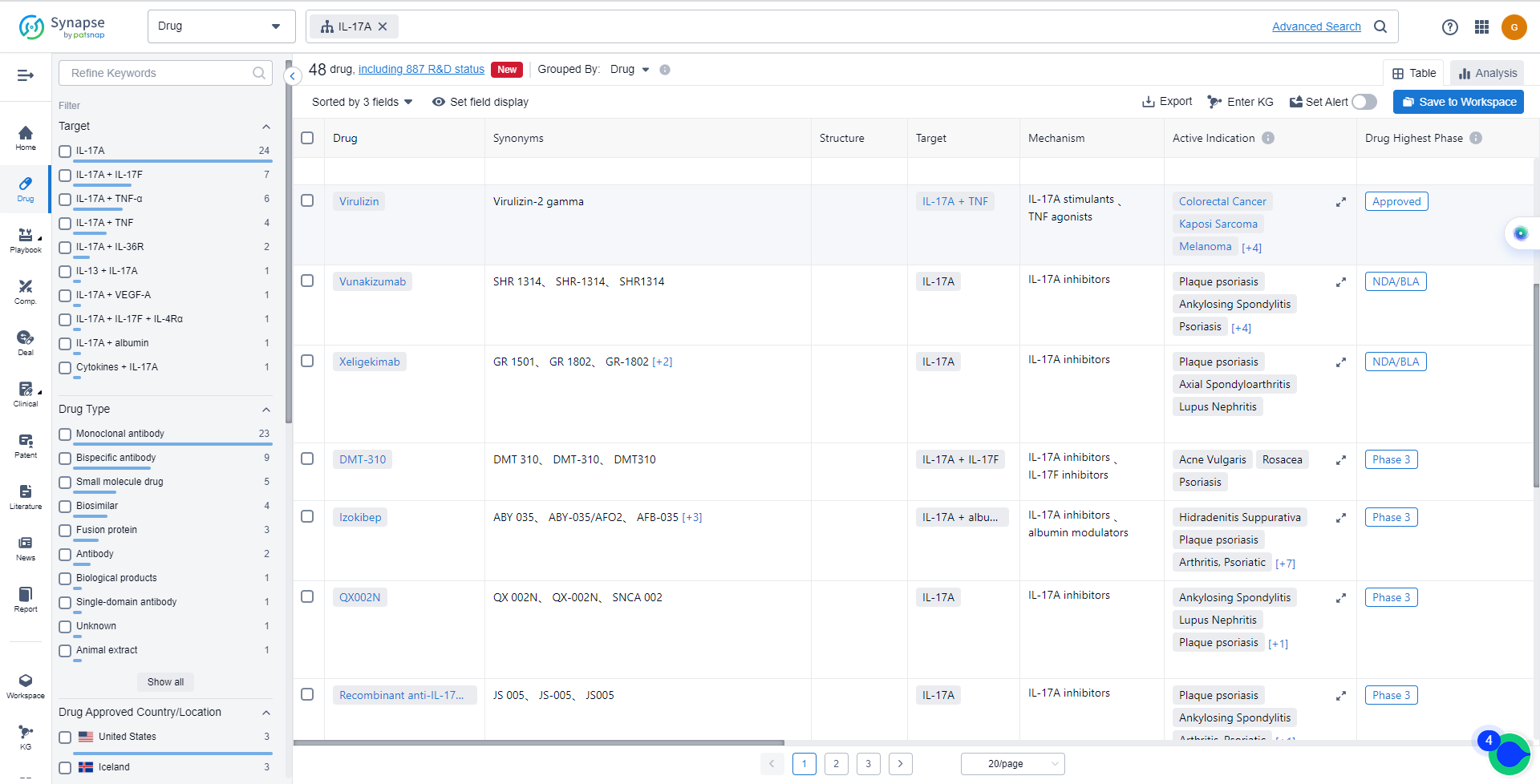
The target IL-17A in the pharmaceutical industry has seen significant growth and development. Novartis AG, UCB SA, and Eli Lilly & Co. are among the companies growing fastest under this target. The highest stage of development is seen in Novartis AG, with drugs in various phases. Drugs under the target IL-17A have been approved for indications such as plaque psoriasis, ankylosing spondylitis, and arthritis psoriatic. Monoclonal antibody is the most common drug type, with biosimilars ranking high. The European Union, United States, and Japan are leading in terms of development, with China also making progress. Overall, the competitive landscape for target IL-17A is intense, with potential for future development and innovation.
How do they work?
IL-17A inhibitors are a type of medication that target and inhibit the activity of interleukin-17A (IL-17A), which is a cytokine involved in the immune system's inflammatory response. From a biomedical perspective, IL-17A inhibitors are used in the treatment of various autoimmune diseases, such as psoriasis, psoriatic arthritis, and ankylosing spondylitis. These medications work by blocking the action of IL-17A, thereby reducing inflammation and alleviating symptoms associated with these conditions. IL-17A inhibitors can be administered through injections or infusions and are typically prescribed for patients who have not responded well to other treatments or have moderate to severe disease activity. It is important to note that IL-17A inhibitors may have potential side effects, including increased risk of infections, so close monitoring and regular follow-up with a healthcare professional are necessary during treatment.
List of IL-17A Inhibitors
The currently marketed IL-17A inhibitors include:
For more information, please click on the image below.
What are IL-17A inhibitors used for?
IL-17A inhibitors have shown promising results in treating serious cases of psoriasis and are also used widely for the treatment of rheumatoid arthritis. For more information, please click on the image below to log in and search.
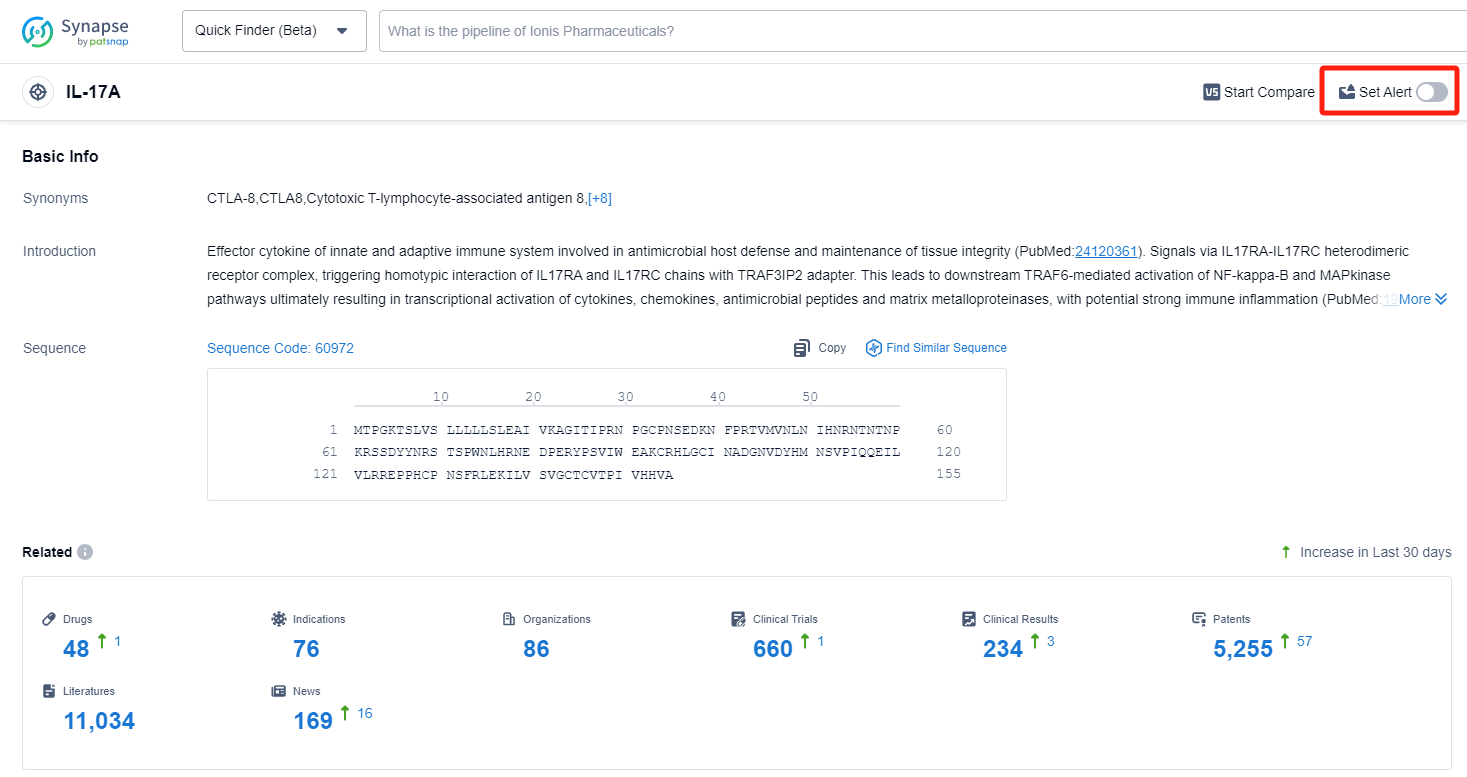
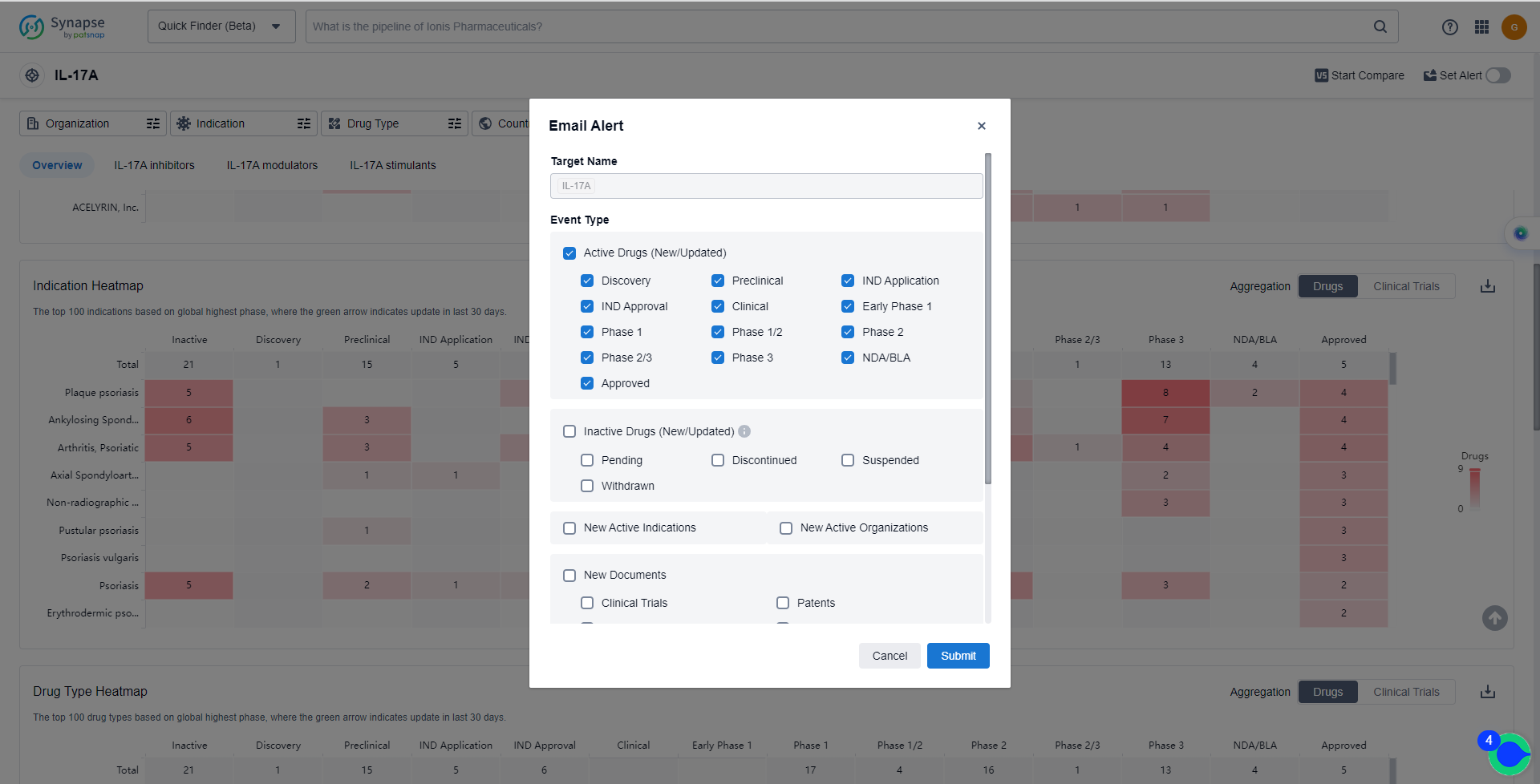
How to obtain the latest development progress of IL-17A inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of IL-17A inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!