European Commission Approves BALVERSA® (Erdafitinib) for Advanced Urothelial Cancer
Janssen-Cilag International NV, a part of the Johnson & Johnson family, has received approval from the European Commission (EC) for BALVERSA®(erdafitinib). This oral medication, taken once daily, is indicated for the treatment of adult patients with unresectable or metastatic urothelial carcinoma (mUC) that have specific fibroblast growth factor receptor 3 (FGFR3) genetic alterations. These patients must have previously undergone at least one therapy regimen that included a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor for their unresectable or metastatic condition.
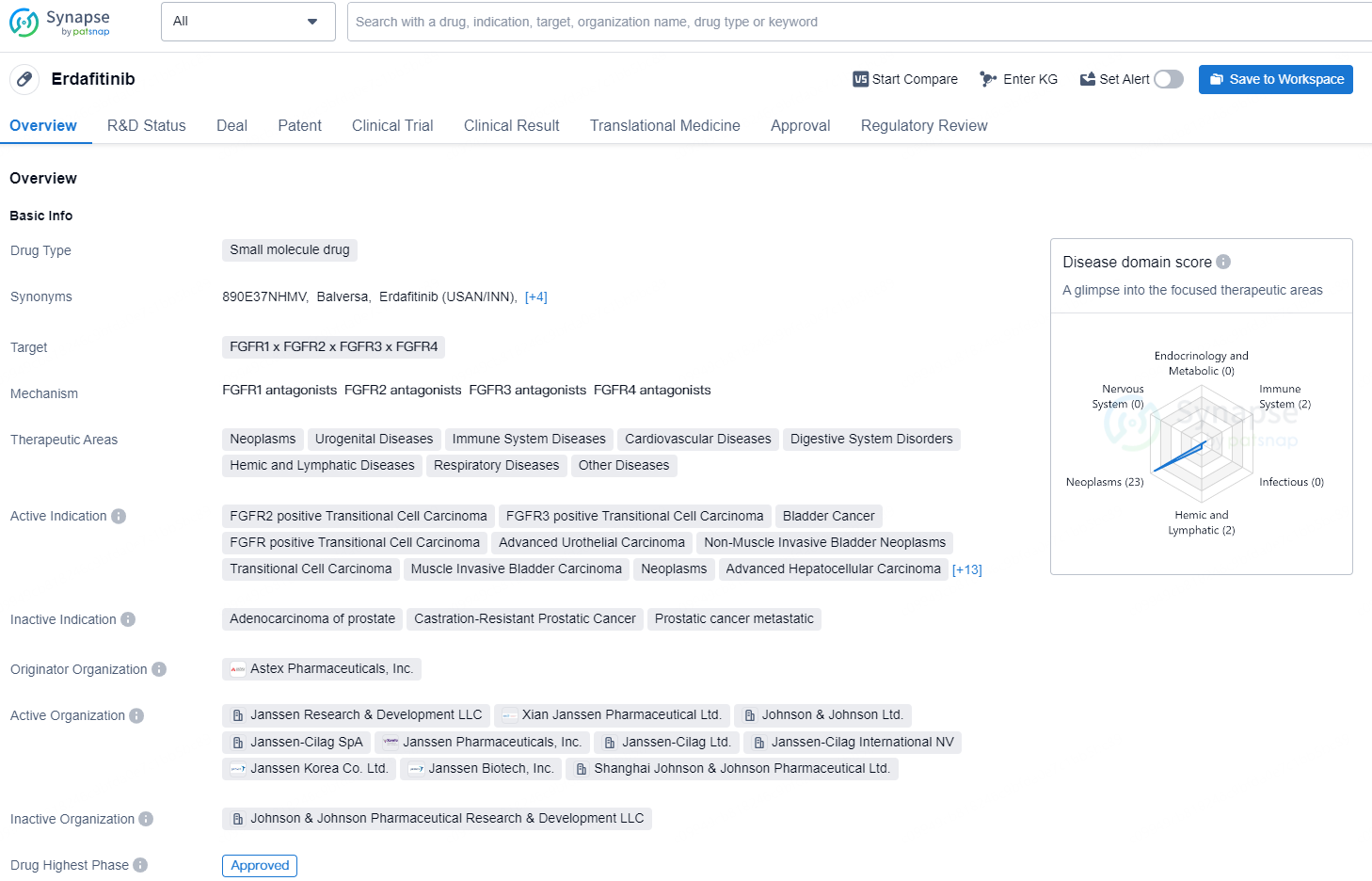
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“Bladder cancer ranks among the most prevalent cancers in Europe, highlighting the critical need for novel treatments for individuals dealing with unresectable or metastatic urothelial carcinoma,” stated Yohann Loriot, M.D., Ph.D., from Institut Gustave Roussy and University of Paris-Saclay, France. “Erdafitinib offers a groundbreaking, targeted therapy that has demonstrated a notable improvement in both overall and progression-free survival for patients with FGFR3 alterations, who previously had very limited treatments available.”
Europe leads the world in bladder cancer cases, with close to 250,000 people diagnosed in 2022, marking a 10 percent rise since 2020. Urothelial carcinoma (UC) constitutes the most prevalent form of bladder cancer, and up to 20 percent of patients with metastatic urothelial carcinoma (mUC) exhibit FGFR alterations. The prognosis for mUC patients remains grim, with a mere eight percent of those diagnosed at an advanced metastatic stage surviving for five years.
“This milestone underscores the crucial role that targeted therapies play in addressing the unique genetic and disease profiles of patients suffering from urothelial carcinoma. It also reinforces our commitment to pioneering precision treatments in oncology,” said Henar Hevia, Ph.D., Senior Director, EMEA Therapeutic Area Lead, Oncology, Johnson & Johnson Innovative Medicine. “The authorization of erdafitinib as a precision therapy accentuates the importance of FGFR testing for all patients with metastatic urothelial carcinoma, as well as the necessity for a multi-disciplinary approach to optimize outcomes for every patient.”
Erdafitinib received EC approval based on findings from Cohort 1 of the Phase 3 THOR study (NCT03390504), which assessed the efficacy and safety of erdafitinib (n=136) compared to chemotherapy (n=130) in patients with advanced or mUC exhibiting specific FGFR alterations, who had progressed following one or two previous treatments, including at least one anti-PD-(L)1 agent.
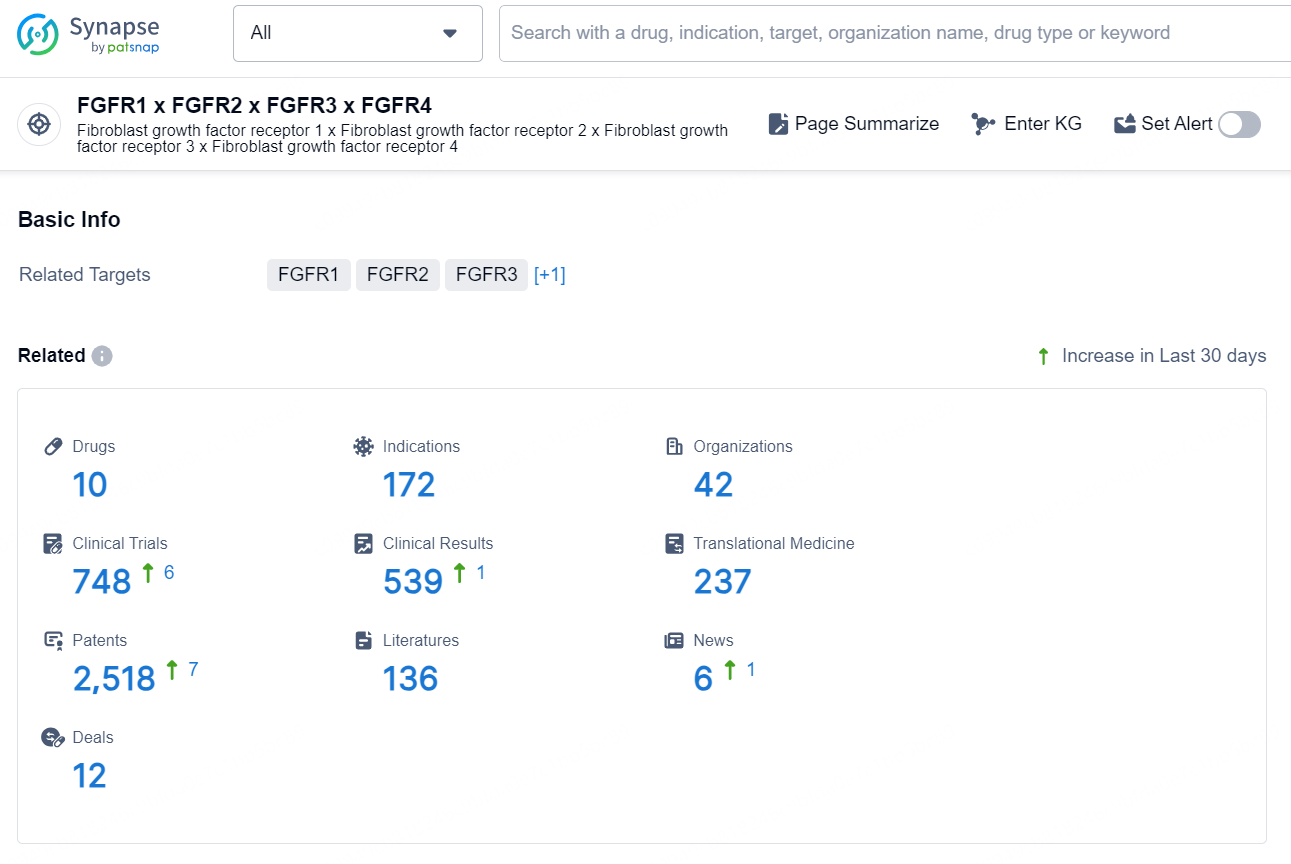
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 26, 2024, there are 10 investigational drugs for the FGFR targets, including 172 indications, 42 R&D institutions involved, with related clinical trials reaching 748, and as many as 2518 patents.
Erdafitinib is a small molecule drug developed by Astex Pharmaceuticals, Inc., which has been approved for use in the United States since April 2019. It is designed to target multiple Fibroblast Growth Factor Receptors (FGFRs), including FGFR1, FGFR2, FGFR3, and FGFR4. The drug has shown potential in treating a wide range of therapeutic areas, including neoplasms, urogenital diseases, immune system diseases, cardiovascular diseases, digestive system disorders, hemic and lymphatic diseases, respiratory diseases, and others.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!