European Commission Approves PADCEV and KEYTRUDA as First-Line Treatment for Advanced Urothelial Carcinoma
Astellas Pharma Inc. (TSE: 4503, President and CEO: Naoki Okamura, "Astellas") revealed that the European Commission has approved Marketing Authorization for PADCEV™ (enfortumab vedotin, an antibody-drug conjugate ADC) in conjunction with KEYTRUDA® (pembrolizumab, a PD-1 inhibitor) for initial treatment in adult individuals with unresectable or metastatic urothelial cancer, who qualify for platinum-based chemotherapy.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The endorsement is predicated on the data from the Phase 3 EV-302 clinical trial, also titled KEYNOTE-A39, which indicated that enfortumab vedotin combined with pembrolizumab almost doubled the median overall survival (OS) and significantly prolonged progression-free survival (PFS) compared to therapies involving platinum-based chemotherapeutics.
Dr. Thomas Powles, Barts Cancer Institute Biomedical Research Centre, UK
“Introducing a new and effective first-line therapy for advanced urothelial carcinoma represents a significant breakthrough in managing this often deadly disease. The notable benefits of the treatment pairing were evident during the Phase 3 clinical evaluations, with enfortumab vedotin along with pembrolizumab markedly enhancing both overall survival and progression-free survival relative to platinum-based chemotherapy. I am excited to witness this treatment combination being adopted as a primary regimen in clinical practice.”
Alex Filicevas, Executive Director, World Bladder Cancer Patient Coalition
“Europe reports the highest incidence of new bladder cancer cases globally, yet awareness is inadequate, leading to many patients receiving accurate diagnoses only at advanced stages of the disease. There is a critical need for new treatment alternatives to improve disease prognosis for these patients and provide optimism for a better future for the bladder cancer patient community worldwide.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
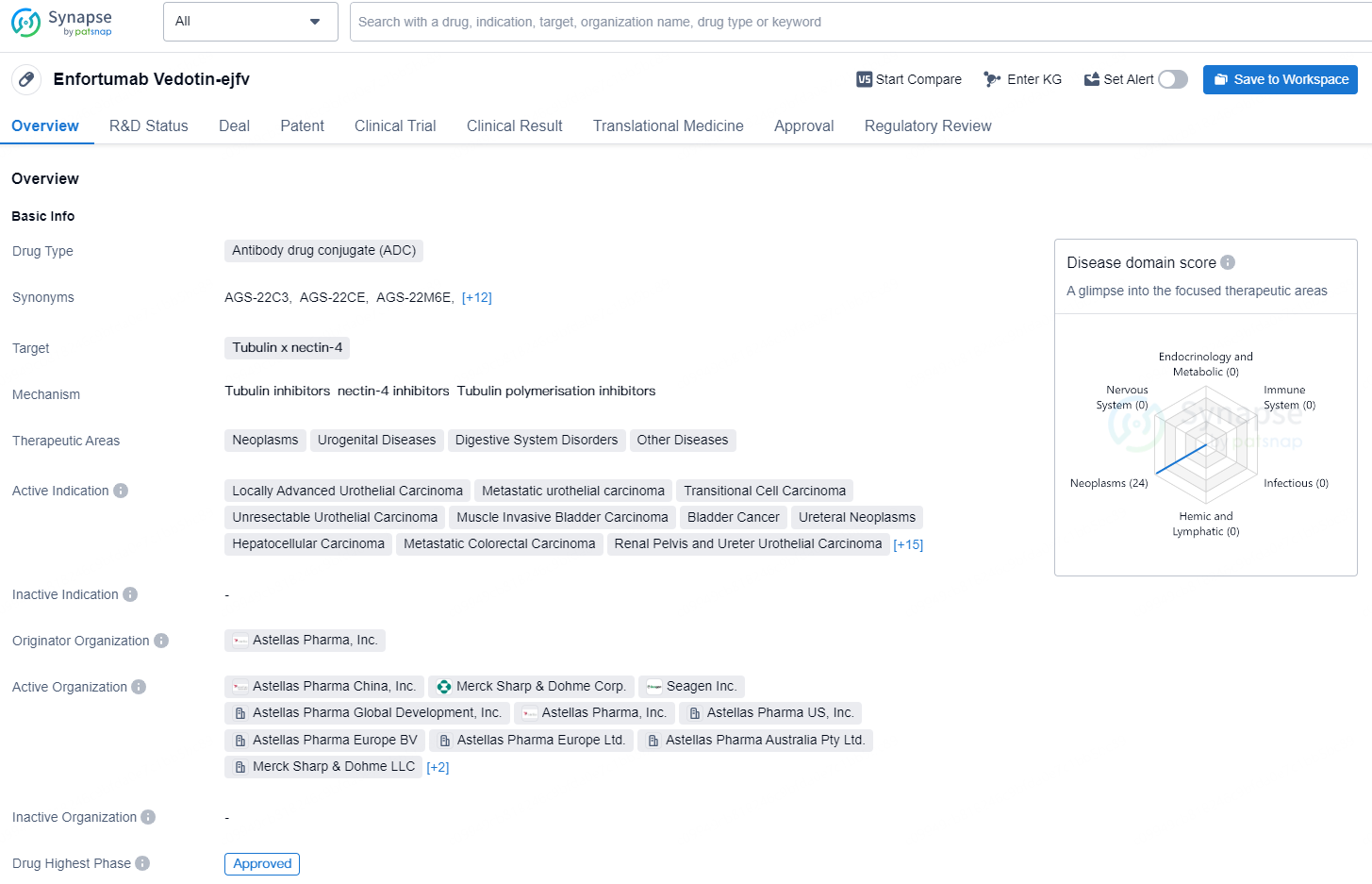
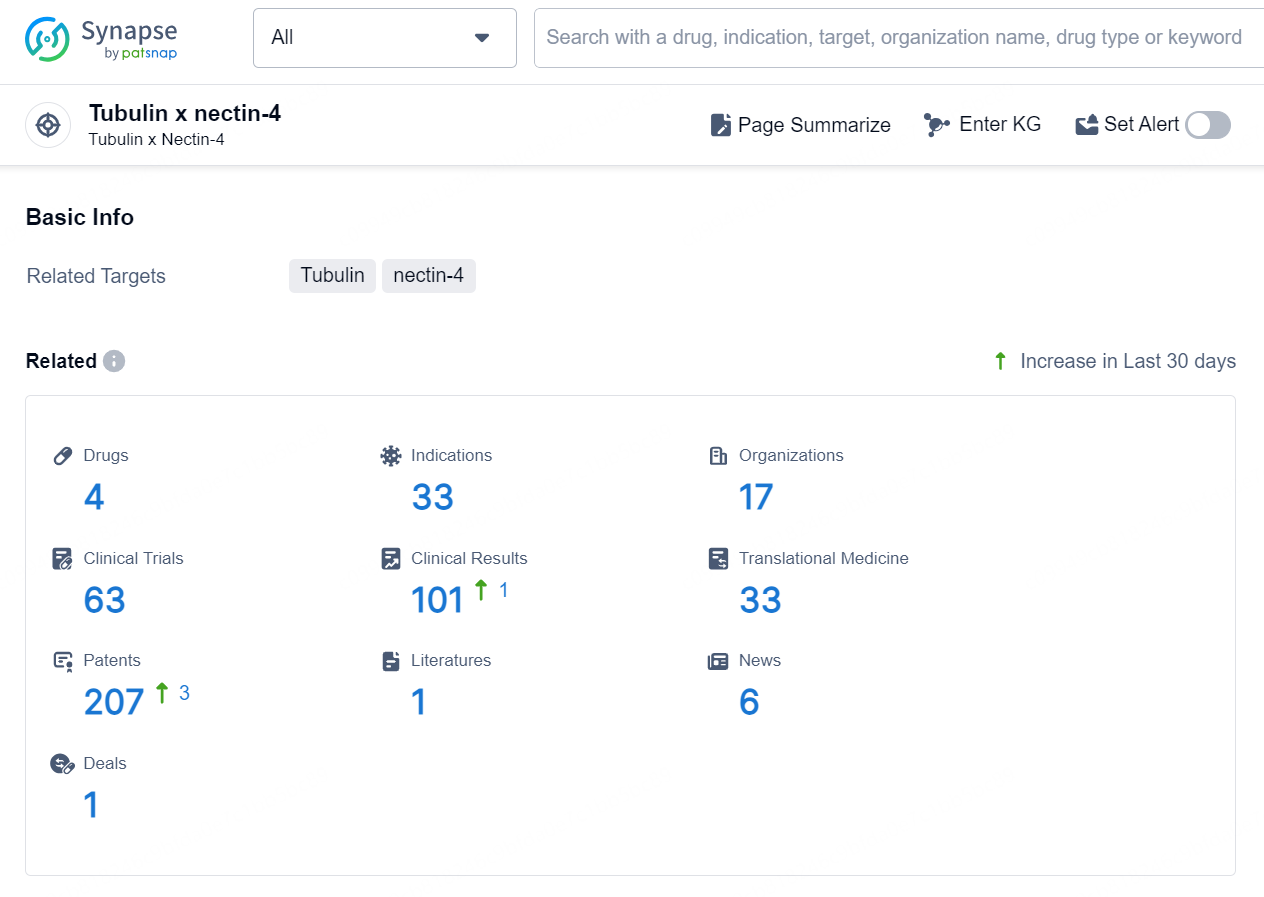
According to the data provided by the Synapse Database, As of August 30, 2024, there are 4 investigational drugs for the tubulin and nectin-4 target, including 33 indications, 17 R&D institutions involved, with related clinical trials reaching 63, and as many as 207 patents.
Enfortumab vedotin-ejfv is an antibody drug conjugate (ADC) that targets tubulin and nectin-4. It has been approved for the treatment of various neoplasms and urogenital diseases, including locally advanced urothelial carcinoma, metastatic urothelial carcinoma, transitional cell carcinoma, unresectable urothelial carcinoma, muscle invasive bladder carcinoma, bladder cancer, ureteral neoplasms, and hepatocellular carcinoma. It has also been approved for the treatment of metastatic colorectal carcinoma, renal pelvis and ureter urothelial carcinoma, urinary retention, bladder squamous cell carcinoma, locally advanced bladder carcinoma, urachal adenocarcinoma, urothelial carcinoma of the urinary bladder, metastatic castration-resistant prostate cancer, advanced urothelial carcinoma, locally advanced malignant solid neoplasm, metastatic solid tumor, renal pelvis carcinoma, urethral neoplasms, carcinoma of urinary bladder invasive, carcinoma in situ, and non-muscle invasive bladder neoplasms.