Evorpacept Enhances Tumor Response in HER2+ Gastric Cancer: ALX Oncology's ASPEN-06 Trial
ALX Oncology Holdings Inc., a firm specializing in immuno-oncology and creating treatments targeting the CD47 immune checkpoint pathway, revealed primary results from its Phase 2 ASPEN-06 clinical study. The results indicated significant clinical advancements in overall response rate and duration of response in patients with HER2-positive advanced gastric cancer or gastroesophageal junction cancer who have been previously treated.
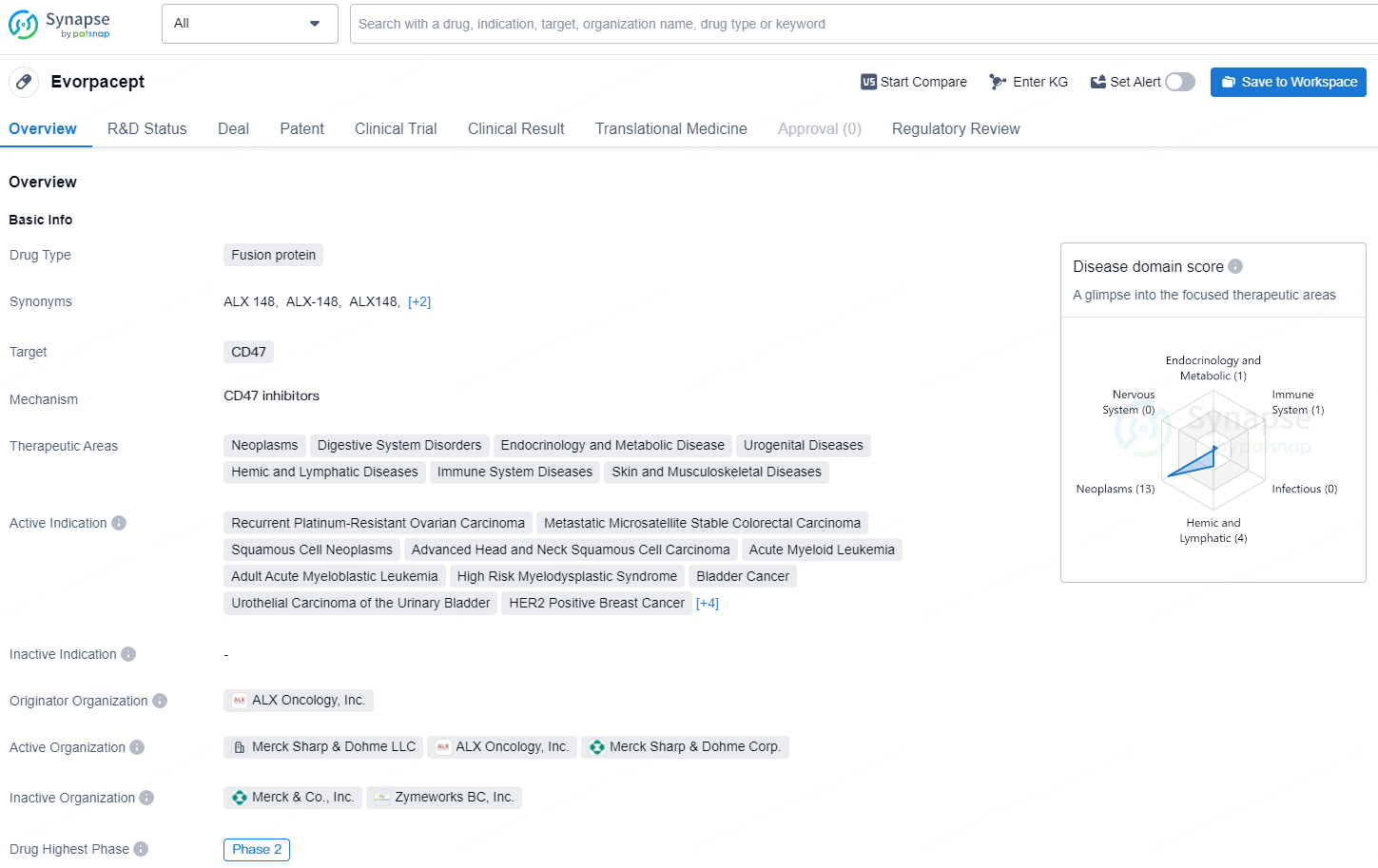
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Results from the ASPEN-06 clinical study affirm that evorpacept can elicit a strong response, significantly impacting key anti-cancer metrics for gastric cancer patients and consistently exceeding field standards," stated Jason Lettmann, CEO of ALX Oncology.
"In addition, these findings extend beyond previously reported interim data, offering a more definitive understanding of evorpacept’s effects and identifying the patient groups that benefit most. The clinical benefits observed in this trial support further development of evorpacept combined with anti-cancer antibodies across other tumor types, guiding ALX's development plans," added Lettmann.
ASPEN-06 is a randomized, multicenter, global trial assessing evorpacept, ALX Oncology’s experimental CD47-blocking agent, which combines a high-affinity CD47-binding element with a proprietary inactivated Fc domain, alongside trastuzumab, CYRAMZA (ramucirumab), and paclitaxel, against a control treatment in patients with HER2-positive gastric/GEJ cancer who had previously been treated with an anti-HER2 therapy. The trial's primary endpoint is overall response rate, with key secondary endpoints including safety, median duration of response, progression-free survival, and overall survival.
"Achieving our clinically significant and predefined benchmark of more than a 10% difference in response between the evorpacept treatment and control groups, these new data substantiate the action mechanism and potential clinical usefulness of evorpacept for patients. Notably, this is the first CD47 blocker shown to provide clinical benefit and a favorable safety profile in a randomized trial," remarked Sophia Randolph, M.D., Ph.D., CMO of ALX Oncology.
The comprehensive data from ASPEN-06 will be presented at a future medical conference.Evorpacept has received Fast Track designation from the U.S. Food and Drug Administration for second-line treatment of patients with HER2-positive gastric or GEJ carcinoma. Moreover, the FDA and the European Commission have granted Orphan Drug Designation for this indication.
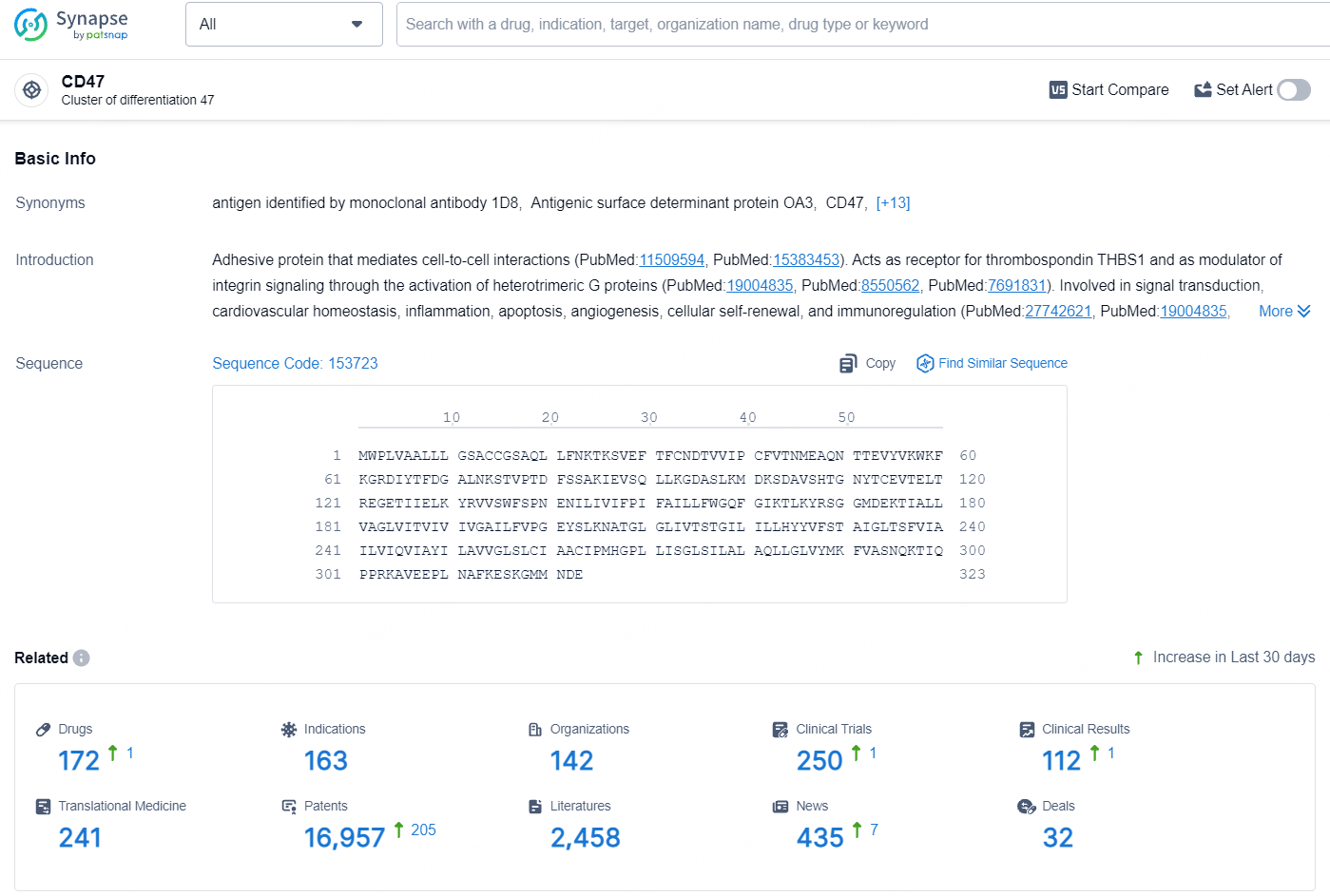
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 7, 2024, there are 172 investigational drugs for the CD47 target, including 163 indications, 142 R&D institutions involved, with related clinical trials reaching 250, and as many as 16957 patents.
Evorpacept holds promise for the treatment of a broad spectrum of cancers and related diseases, with its unique fusion protein targeting CD47. As it advances through the development pipeline, it is positioned to potentially address critical unmet medical needs for patients with the specified indications.