Is Etrasimod approved by the FDA?
Yes, etrasimod (Velsipity) is FDA approved. The FDA granted its approval on October 12, 2023. This approval allows for the use of etrasimod in the treatment of adults with moderately to severely active ulcerative colitis.
Usage and Administration
Etrasimod is administered orally in the form of a 2 mg tablet. The recommended dosage is 2 mg once daily, which can be taken with or without food. It is crucial to follow the prescribed dosage and guidelines provided by the healthcare provider.
Safety and Side Effects
Common side effects of etrasimod include headache, abnormal liver function tests, and dizziness. More serious side effects can include slow heartbeats, chest pain, shortness of breath, severe infections, liver problems, severe vision issues, and skin changes. It's important to seek emergency medical help if you experience any signs of an allergic reaction or serious side effects.
Precautions
Before starting etrasimod, patients should inform their healthcare provider about any existing health conditions, medications, or allergies. Etrasimod can slow heart rate when starting treatment and may increase the risk of infections, including serious or fatal infections. Patients should also be aware of the increased risk of skin cancer and liver problems associated with etrasimod use.
Etrasimod should not be used by individuals with certain serious heart conditions or if they have had severe heart issues within the past six months. Pregnant women and those planning to become pregnant should consult their doctor, as etrasimod may harm an unborn baby. Effective birth control is recommended while using etrasimod and for at least seven days after the last dose.
Storage and Handling
Etrasimod should be stored at room temperature, away from moisture and heat. The tablets should be kept in their original container and protected from light.
Conclusion
Etrasimod (Velsipity) is an FDA-approved medication for the treatment of moderately to severely active ulcerative colitis in adults. Approved on October 12, 2023, it offers a new option for managing this chronic inflammatory condition. Patients prescribed etrasimod should follow their healthcare provider’s instructions carefully and be aware of potential side effects and precautions to ensure safe and effective use. Always consult with a healthcare professional for personalized medical advice.
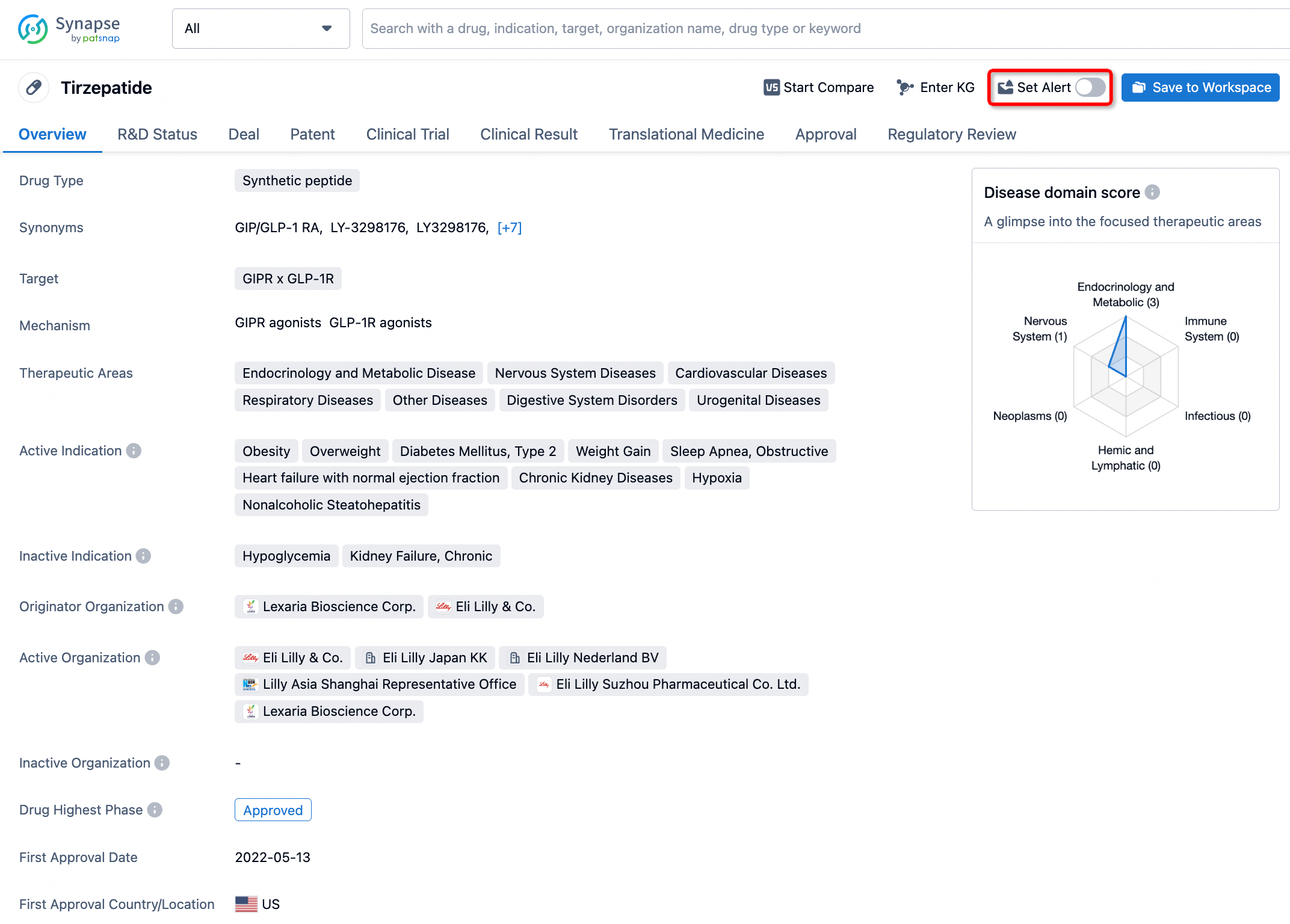
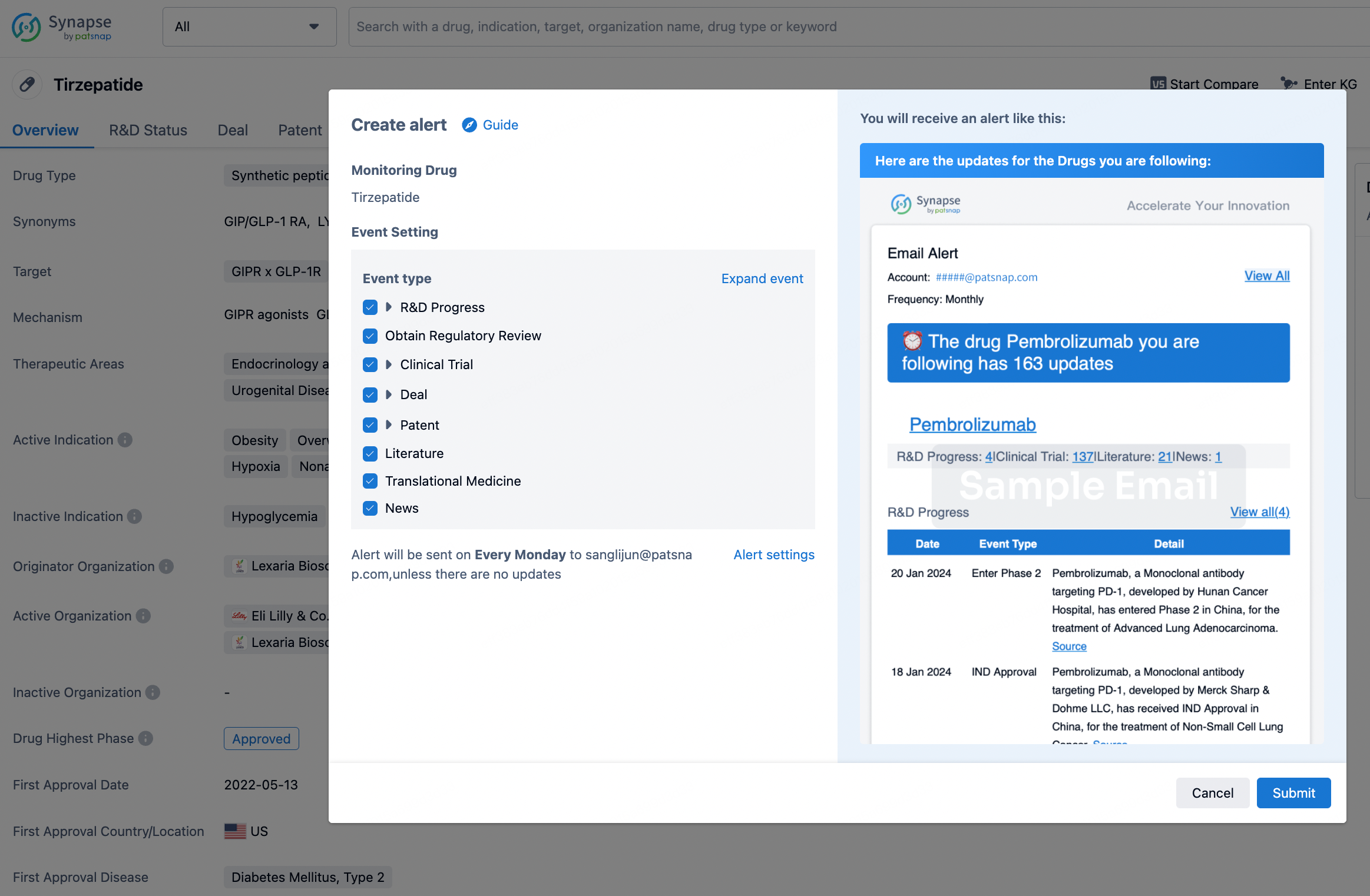
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!