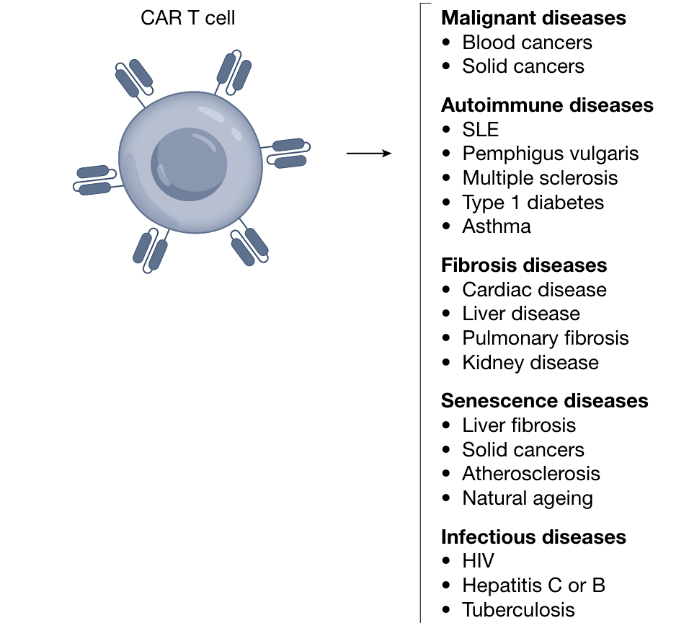
Expanding the Horizons of CAR T Therapy Beyond Cancer Treatment: Part I
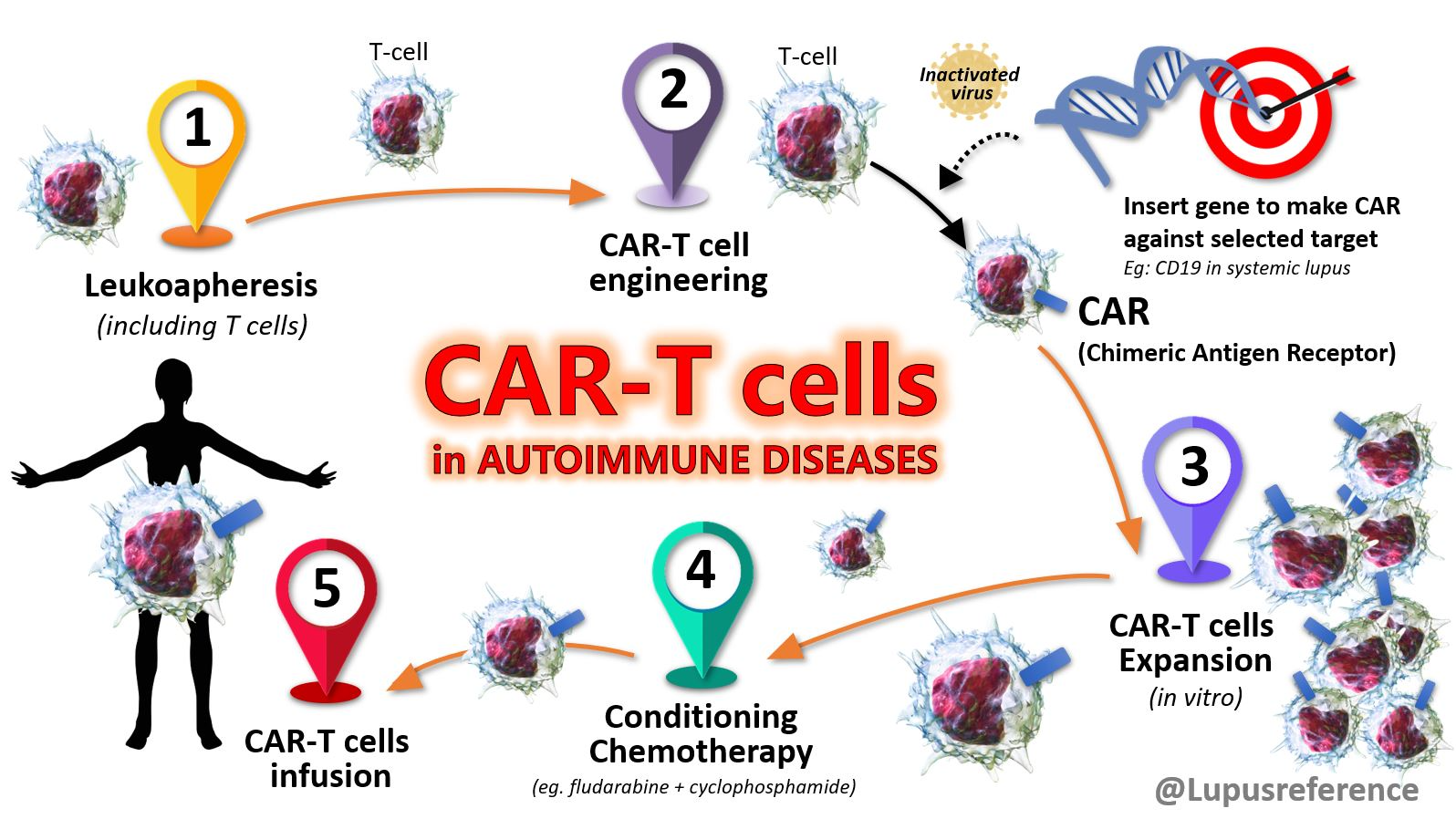
Over the past decade, CAR-T cell therapy has transformed tumor treatment and brought new hope to cancer patients. In fact, CAR-T was not originally intended for cancer but to redirect T cells to eliminate HIV. Although early experiments were disappointing, continuous optimization (adding co-stimulatory domains) has enabled CAR-T to demonstrate unprecedented efficacy compared to conventional therapies in treating relapsed refractory hematologic malignancies. These clinical successes continue to drive adoptive cell therapy development. As research has progressed, CAR-T’s efficacy has been found to extend beyond cancer with great potential in treating autoimmunity, infections, fibrosis, aging-related diseases and more.
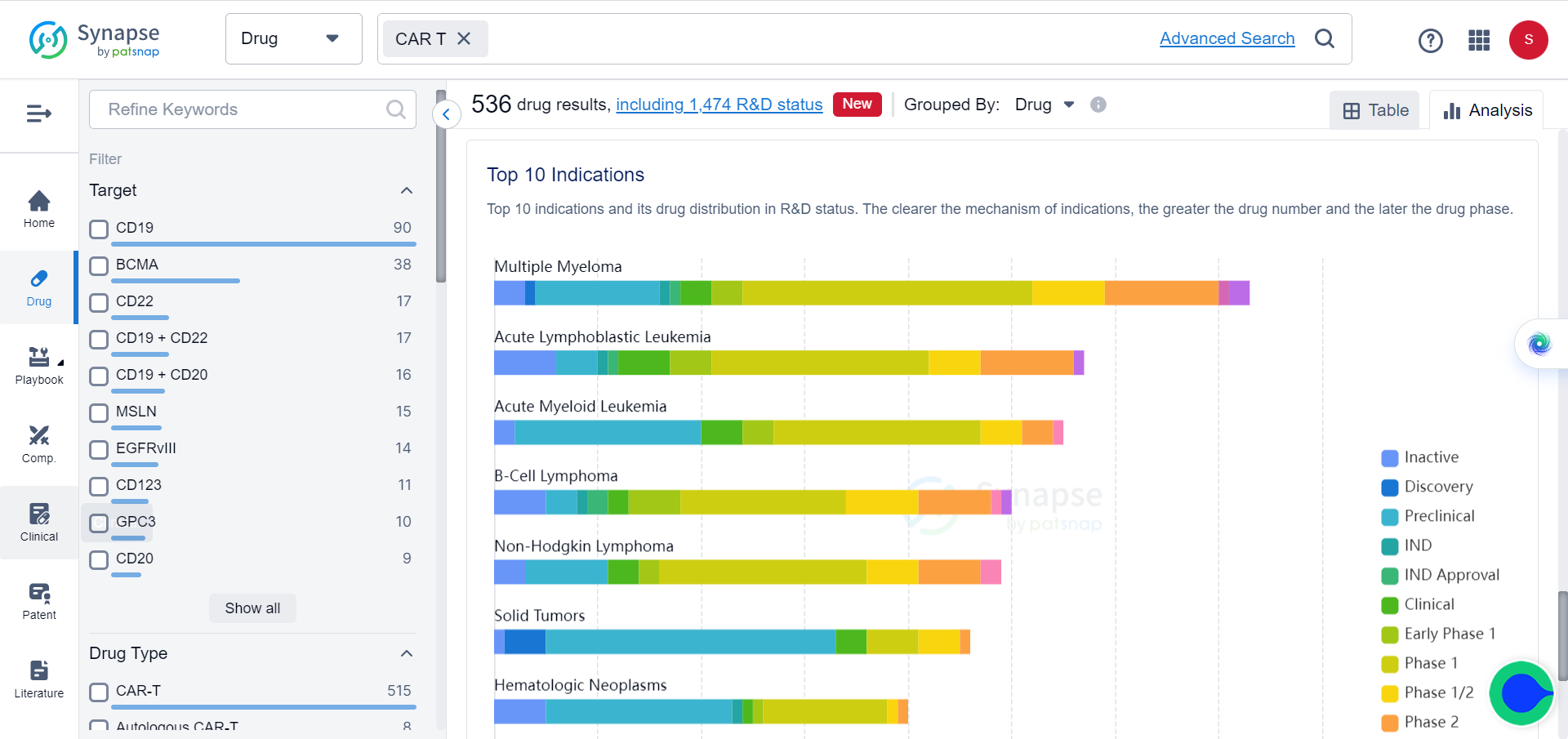
CAR-T applications in non-cancer diseases
Years of clinical trials demonstrate that eliminating specific cell populations has therapeutic effects achievable through customized CAR-T cells. This has expanded research beyond cancer into other refractory diseases caused by specific pathologic cell types. Recent clinical and preclinical results suggest that CAR-T cells may be effective in certain autoimmune diseases, infections, heart failure and chronic conditions by eliminating pathological cells.
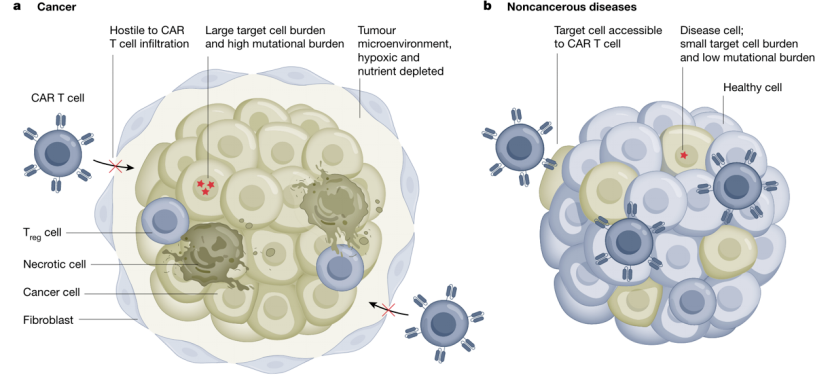
Treating non-cancer diseases might be an easier task for CAR-T than solid tumors. In tumor treatment, larger doses of CAR-T are often required, which increases the risk of cytokine release syndrome. In contrast, targeting cells in other diseases may require relatively few doses.
In cancer treatment, complete elimination of malignant cells is necessary to prevent relapse, whereas partial removal of pathological cells may suffice in other diseases. Additionally, tumors often mutate to escape antigens, while other diseases lack this capacity. Most importantly, solid tumors possess barriers and immunosuppression that obstruct CAR-T cells, which other diseases lack. This enables CAR-T cells to more easily clear pathological cells in non-cancer diseases. These factors have contributed to the rapid application of CAR-T cell therapy in non-cancer diseases.
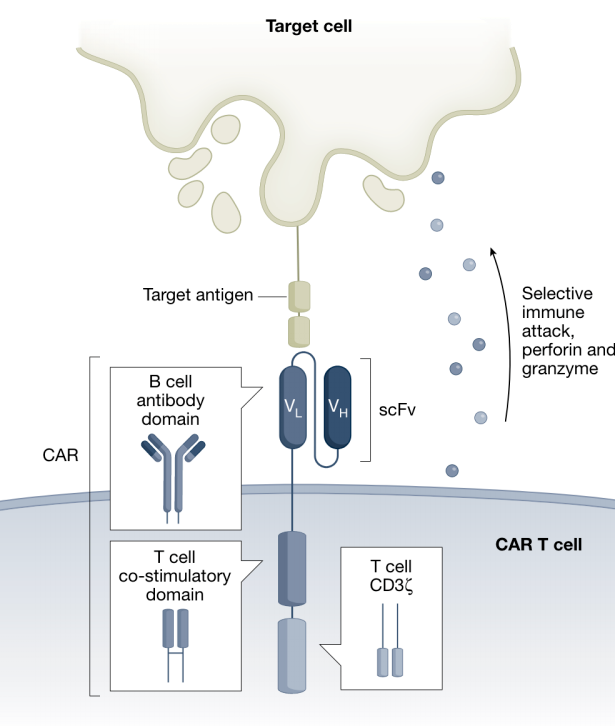
Autoimmune inflammatory diseases
In systemic lupus erythematosus (SLE), an autoimmune disorder that poses a serious threat to life, the injection of anti-CD19 CAR-T into six patients in 2021-2022 resulted in CAR-T expansion, rapid depletion of B cells, symptom relief, and a decrease in organ damage markers. All patients achieved drug-free remission after discontinuing immunosuppressants. The reemergence of naive B cells several months after infusion without recurrence indicates the treatment's tolerability with only mild cytokine release syndrome (CRS), which is consistent with reduced severity due to a lower antigen burden.
Anti-CD19 CAR-T has also shown effectiveness in easing inflammatory myopathies associated with antisynthetase syndrome. Although patients experienced worsening pain and elevated creatine kinase levels following infusion, significant physical improvement and resolution of muscle inflammation occurred at 180 days, with normalization of biomarkers.
In fact, CAR-T therapy may be used to treat autoimmune diseases mediated by B cells or plasma cells, such as vasculitis, arthritis, myositis, multiple sclerosis, pemphigus, immune thrombocytopenia, myasthenia gravis, and neuromyelitis optica. All the B/plasma cell targets, including BCMA, GPRC5D, CD20, and CD22, can potentially be used.
Alternatively, engineered Tregs redirected to target tissues can be used to eliminate inflammatory cells. These non-cytotoxic cells suppress inflammation and autoimmunity through paracrine signals. CAR-Tregs can increase specificity while reducing the risk of systemic immunosuppression. Current studies indicate that autoimmune diseases may be among CAR-T's most promising applications.
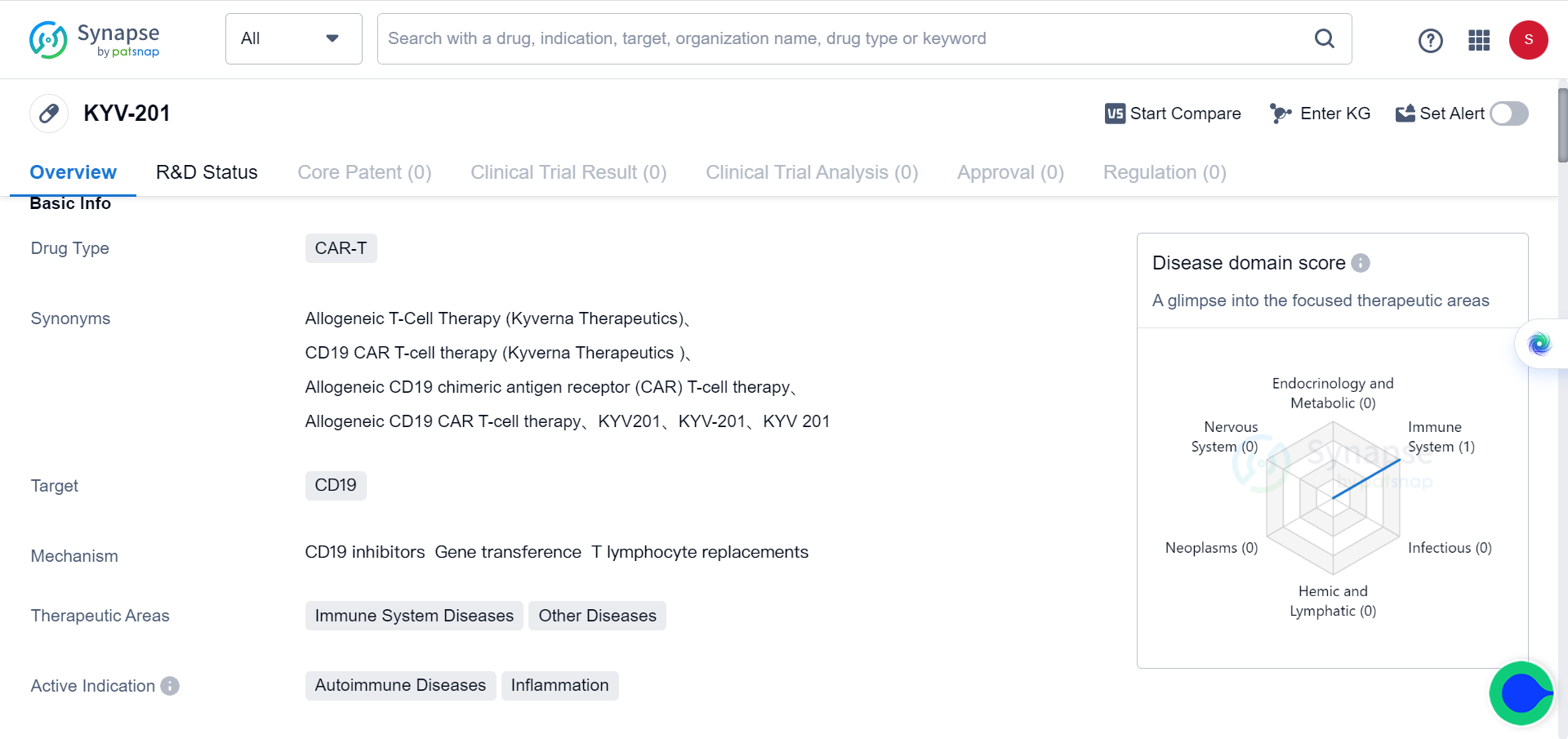
In Synapse database, there’s one drug (KYV-201) that’s currently in preclinical stage of development for autoimmune disease & inflammation.
Aging
Various stressors can trigger irreversible cell growth arrest, known as senescence, which contributes to chronic disease. Removing senescent cells has been shown to benefit chronic disease models. While intracellular pathway inhibitors show promise, issues with efficacy, specificity, and side effects persist. In contrast, CAR-T therapy, with its inherent specificity and potency, is a highly competitive senolytic therapy. Enhancing the physiological immune clearance of senescent cells early in life with CAR-T could yield significant health benefits.
Recent studies have shown that CAR-T therapy can eliminate senescent cells in mouse models of cancer and liver disease. The urokinase plasminogen activator receptor (uPAR), encoded by PLAUR and thought to be a potential serum antigen with low expression in vital tissues, was targeted by anti-uPAR CAR-T cells, which successfully cleared senescent cells induced by oncogenes, tumor therapy, drugs, and diet in mice. Identifying senescence antigens is challenging due to heterogeneity, but it is important. Other potential targets include NKG2D ligands MICA, MICB, and ULBP1-5, which are expressed across multiple senescent cells, and GPNMB, an immunotherapy target that removed senescent cells, improved metabolism, reduced atherosclerosis, and extended lifespan in progeroid mice, suggesting its utility as a senescence CAR-T target.
Infection
Targeting non-self antigens greatly reduces the risk of off-tumor toxicity. As mentioned earlier, CAR-T was originally developed to treat HIV patients. After viral infection, host cells display specific antigens on their surface that CAR-T cells designed against these antigens could potentially clear. With the optimization of CAR-T structures, second-generation CAR-T has also been applied in HIV treatment, with clinical trials underway. Fungal pattern recognition receptor-based CAR-T cells recognizing carbohydrate antigens in fungal cell walls have shown antifungal properties in vitro and in mouse models. Similarly, a recent study tested CAR-T efficacy against invasive pulmonary aspergillosis, demonstrating antifungal effects in vitro and in mouse models. While CAR-T shows promise in chronic infections, the current ex vivo production cannot provide the urgent response needed for acute infections.
Organ fibrosis
Organ fibrosis, such as cardiac fibrosis from injury, disease, or aging, raises morbidity and mortality rates. Treatment options are limited, but CAR-T therapy shows promise in targeting fibrosis and restoring function after hypertension injury in preclinical studies. Researchers identified fibroblast activation protein (FAP), highly expressed in cardiomyopathy patients, as a key antigen. Anti-FAP CAR-T cells resolved post-injury fibrosis and improved cardiac function. The anti-fibrotic potential extends beyond the heart, as extracellular matrix deposition and fibrosis are pathological in many diseases. Liver, kidney, lung, muscle diseases, and more may benefit from anti-fibrotic CAR-T therapy.
CAR-T clinical and preclinical studies across non-cancer diseases suggest that this treatment can cure patients by eliminating specific pathological cell populations, providing more thorough and sustained efficacy compared to small molecules and monoclonal antibodies. These results indicate that CAR-T therapy may open up new therapeutic frontiers beyond cancer.

Reference
Baker DJ, Arany Z, Baur JA, Epstein JA, June CH. CAR T therapy beyond cancer: the evolution of a living drug. Nature. Jul 2023;619(7971):707-715.