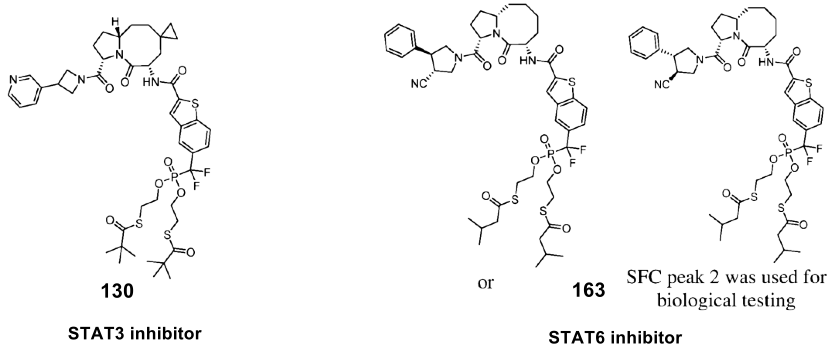
The Discovery Process of a Novel STAT6 Inhibitor
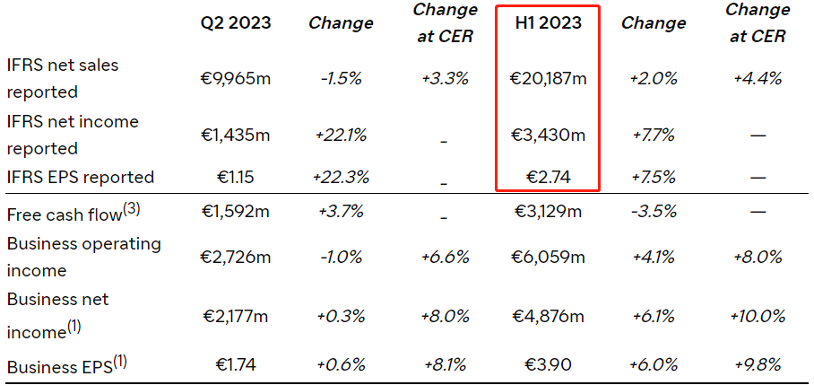
On July 28, 2023, Sanofi Releases Q2 Financial Report, with H1 Revenue of 20.187 Billion Euros, a year-on-year growth of 2%. Its heavy-weight product, the IL4/IL13 Antibody Dupixent Achieved H1 Sales of 5.35 Billion US Dollars, Expected to Break the 10 Billion Dollar Mark for the First Time This Year.
Dupixent is a fully humanized monoclonal antibody that inhibits the signal transmission of the IL-4 and IL-13 pathways. It is a flagship product of Sanofi's Th2 pathway. Dupixent has been approved by the FDA to treat diseases including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, nodular prurigo, and eosinophilic esophagitis. However, the treatment for allergic asthma, pollen allergy, and peanut allergy is at a standstill or terminated stage.
Sanofi is continuously strategizing for the Th2 pathway and frequently making acquisitions. On 20th July 2023, Sanofi announced its collaboration with Recludix Pharma, licensing the latter's globally pioneering autoimmune drug, the STAT6 inhibitor. According to the agreement, Sanofi will make an upfront payment of $125 million, milestone payments of up to $1.2 billion, and double-digit sales royalties. Recludix is responsible for advancing development until the commencement of Phase II trials, after which Sanofi will take over subsequent clinical development and commercialization.
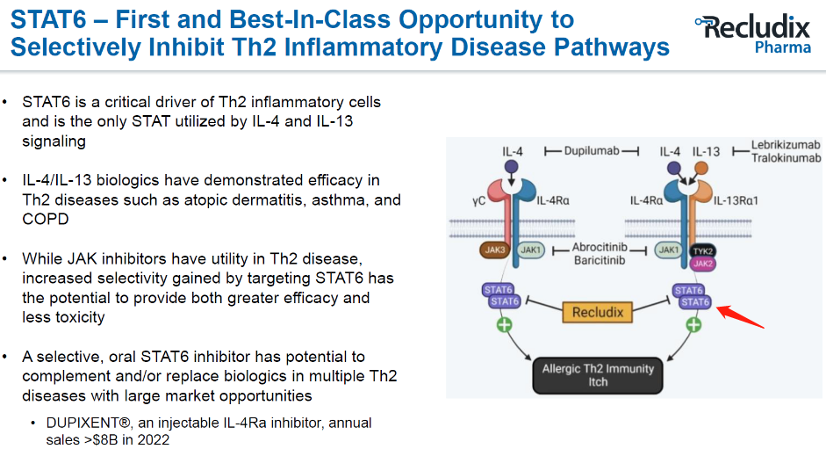
According to information on Recludix's official website, STAT6 is essential for IL-4 and IL-13 signal transduction, but it is downstream of the JAK/STAT pathway and is not utilized by other cytokines and growth factors. Therefore, selective STAT6 inhibitors are considered to have stronger specificity and fewer side effects. Recurrent, activated hotspot mutations in STAT6 have been identified in B-cell lymphoma, with approximately 5% to 30% of patients with these diseases carrying STAT6 mutations. Thus, STAT6 inhibitors also have potential therapeutic effects for these tumor patients.
Signal transducer and transcription activator (STAT) is a type of cytoplasmic transcription factor responsible for the transduction of signals from extracellular cytokines and growth factors and the activation of gene transcription. In mammalian cells, the STAT family consists of 7 members, including STAT1, STAT2, STAT3, STAT4, STAT5α, STAT5β, and STAT6, with homology ranging from 20% to 50%. Many genes regulated by STAT proteins include those involved in controlling the cell cycle, cell survival, and immune responses.
Recludix, a very young company established in 2021, was co-founded by Nicholas Lydon, the scientific founder of Blueprint Medicines. The only public patent of Recludix currently is WO2023133336A1, which discloses a type of STAT3 and/or STAT6 modulators on July 13, 2023, for treating various diseases related to STAT3 and/or STAT6. The primary priority date for the patent WO2023133336A1 is January 10, 2022, with an international application time one year later, and it was published 18 months later. The PCT designated deadline is September 10, 2024, it has not yet entered China. The patent includes activity data of 1127 compounds with STAT3/STAT6, expressed by letters in terms of potency.
When searching for STAT6 on Synapse, only antisense oligonucleotide CDK 004 is in phase I clinical trial for colorectal cancer and other conditions; in addition to Recludix's REX-2787 and REX-4671, main preclinical candidates include PM-43I and CLXR-005, as well as Astellas' AS1517499, AS1617612, and AS1810722. Astellas' three inhibitors were first discovered in 2009 and have remained in preclinical stage without further progress.
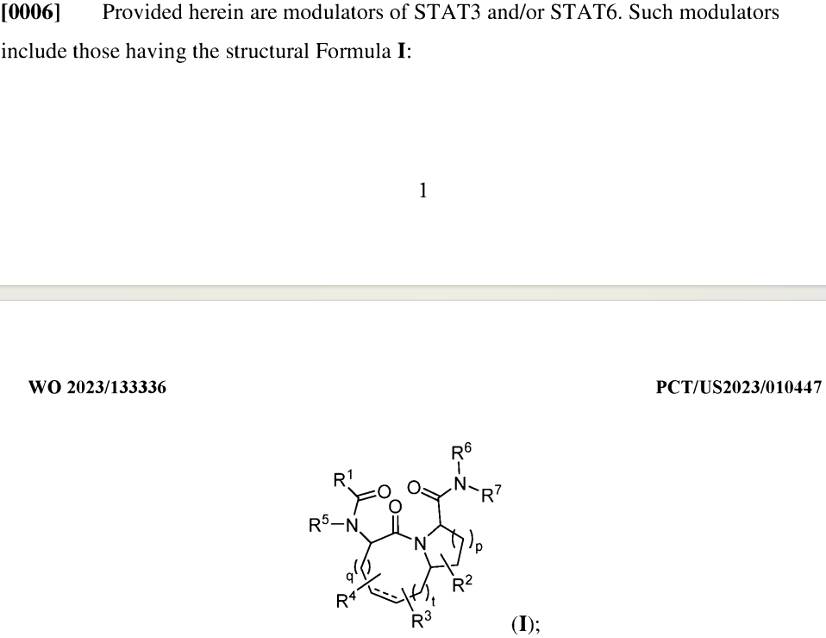
So, where does this amide structure in the patent come from? Although the activity data in the patent is represented by ABCD (A strongest, D weakest), one can still find many STAT3 or STAT6 selective inhibitors based on the SAR in the patent by modifying the steric hindrance on the octahedron and controlling the substituents on the four-nitrogen or five-nitrogen ring, thus synthesizing examples 130 and 163. This achieves regulation of STAT3 or 6, and the patent does not contain suppression data for other subtypes. To explain the characteristics of these molecules, we need to trace back to 2003.
In 2003, McMurray and his colleagues discovered a class of peptide fragments that showed high affinity for the STAT3 protein. The most effective fragment is a hexapeptide (pYLPQTV) derived from residues 904-909 of the gp130 receptor protein, with an IC50 of 150 nM in EMSA experiments. Subsequently, McMurray and Shaomeng Wang independently modified this sequence to provide peptidomimetic analogs with enhanced STAT3 affinity. Specifically, McMurray et al. developed tetrapeptides 3 and 4, which bind tightly to the STAT3 SH2 structural domain, with IC50 values of 125 nM and 17 nM respectively. However, due to poor metabolic stability and limited cell permeability, compounds 3 and 4 showed limited in vivo activity. Subsequent efforts have been directed towards reducing the peptidic properties of these molecules.
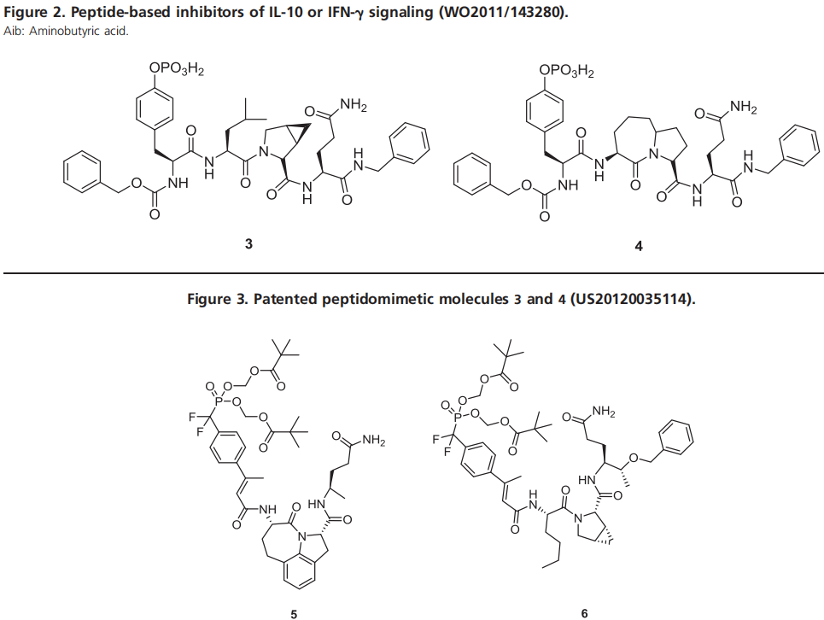
To diminish the peptidic nature of inhibitors, modifications include replacing the pY group with a 4-phosphocinnamic acid moiety, solidifying the relatively hydrophobic core with tricyclic lactam or methylene proline, substituting glutamine replacements, and masking the function of negatively charged phosphates with biologically unstable penta-[methoxy]methoxy (POM) groups. Derivatives of 3 generated 5 and 6, enhancing their cellular activity. In time-dependent inhibition studies on MDA-MB-468 breast cancer cells, 5 and 6 completely abolished the phosphorylation of STAT3 within 30 minutes at 5µM, an effect that lasted for 4 hours, although pSTAT3 fully recovered after 16 hours. POM groups can improve the membrane permeability of the molecule, thus enhancing cellular activity. However, no studies have been reported on the PK/ADME of these molecules to determine the in vivo half-life of the peptide, and the experimental results do not rule out off-target effects.
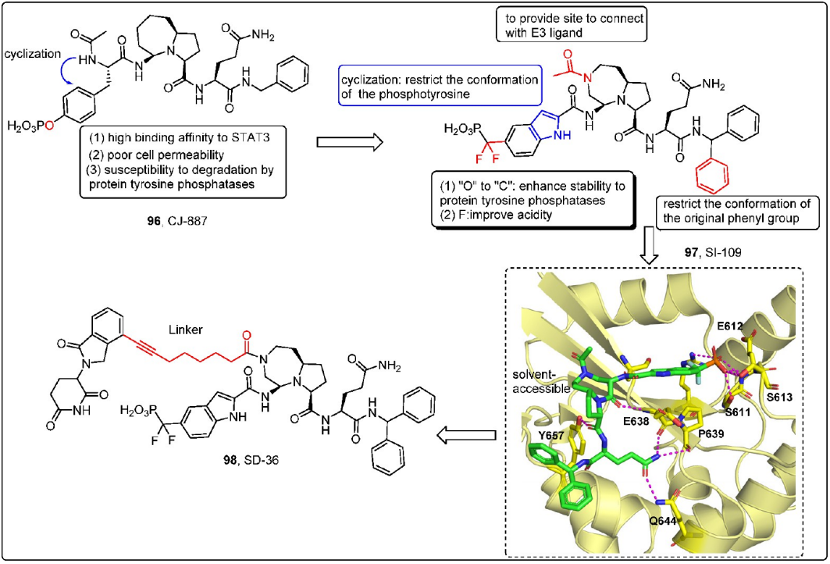
In addition, the research group led by Shaomeng Wang has been exploring ways to highly target STAT3 inhibition. It is challenging to target the SH2 region without affecting STAT1, the normal function of which is beneficial for life organisms. A 2021 JMC review mentioned the STAT3 PROTAC developed by Shaomeng Wang. The small molecule CJ-887 has a high affinity for STAT3, but it has poor cell membrane permeability and is easily degraded by protein tyrosine phosphatase. The oxygen substitution with carbon can increase enzyme stability, and the addition of difluoride can enhance activity. Cyclization into indole or thiazole is also aimed at increasing enzyme stability. The Octahedral exposed amine of SI-109 is determined by the co-crystal structure of SI-109 within a complex having a STAT3 SH2 structural domain, suggesting that it is an appropriate site for linking E3 ubiquitin ligase ligands.
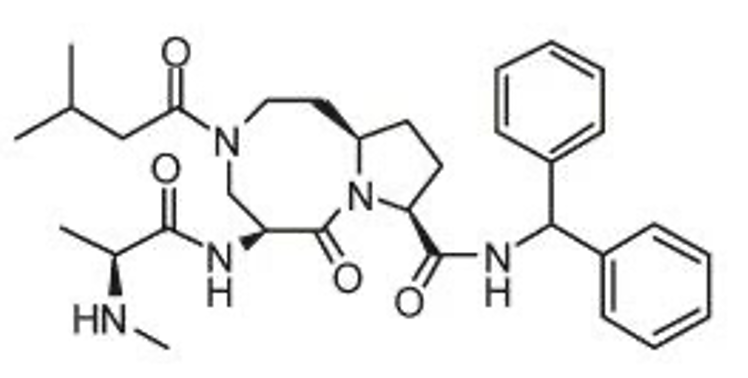
Shaomeng Wang seems to have a particular fondness for peptide-like inhibitors. On March 1, 2021, Germany's Merck announced that the company has acquired the global exclusive rights to Debiopharm's oral experimental drug IAP antagonist xevinapant (Debio1143, AT-406) for nearly 900 million Euros. This includes the development rights to preclinical and post-clinical compounds of xevinapant (Debio1143,AT-406), which also has a peptide-like structure, including segments of penta and octa amide macrocycles. Xevinapant is a potential "first-in-class" drug. Besides the ongoing Phase III clinical trials for squamous cell carcinoma, there are several clinical trials for the treatment of solid tumors in progress, including non-small cell lung cancer, ovarian cancer, and head and neck tumors.
In conclusion, based on the available public information, the STAT6 inhibitor acquired by Sanofi is likely to be modified from a peptide-like inhibitor. STAT6 is downstream of the JAK/STAT pathway, is less disturbed by cytokines and growth factors, and participates in limited pathways. Inhibitors highly targeted at STAT6 may have a higher safety profile compared to JAK1-3 or TYK2. However, STAT3 inhibitors targeting SH2 have not been able to reach the market, and there are very few STAT6 inhibitors in clinical use. Patients do not need drugs that are only as safe as placebos, and whether STAT6 inhibitors are effective still needs to be confirmed by clinical trial POC. We look forward to having more safe and effective drugs for the benefit of patients.

Reference
1.Patrick T Gunning et.al; A STAT inhibitor patent review: progress since 2011. DOI:10.1517/13543776.2015.1086749.
2.Jiang-Jiang Qin et al;Recent Update on Development of Small-Molecule STAT3 Inhibitors for Cancer Therapy: From Phosphorylation Inhibition to Protein Degradation.J. Med. Chem. 2021, 64, 8884−8915.
3.WO 2023/133336