Exploring cabotegravir/rilpivirine's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Cabotegravir/rilpivirine's R&D Progress
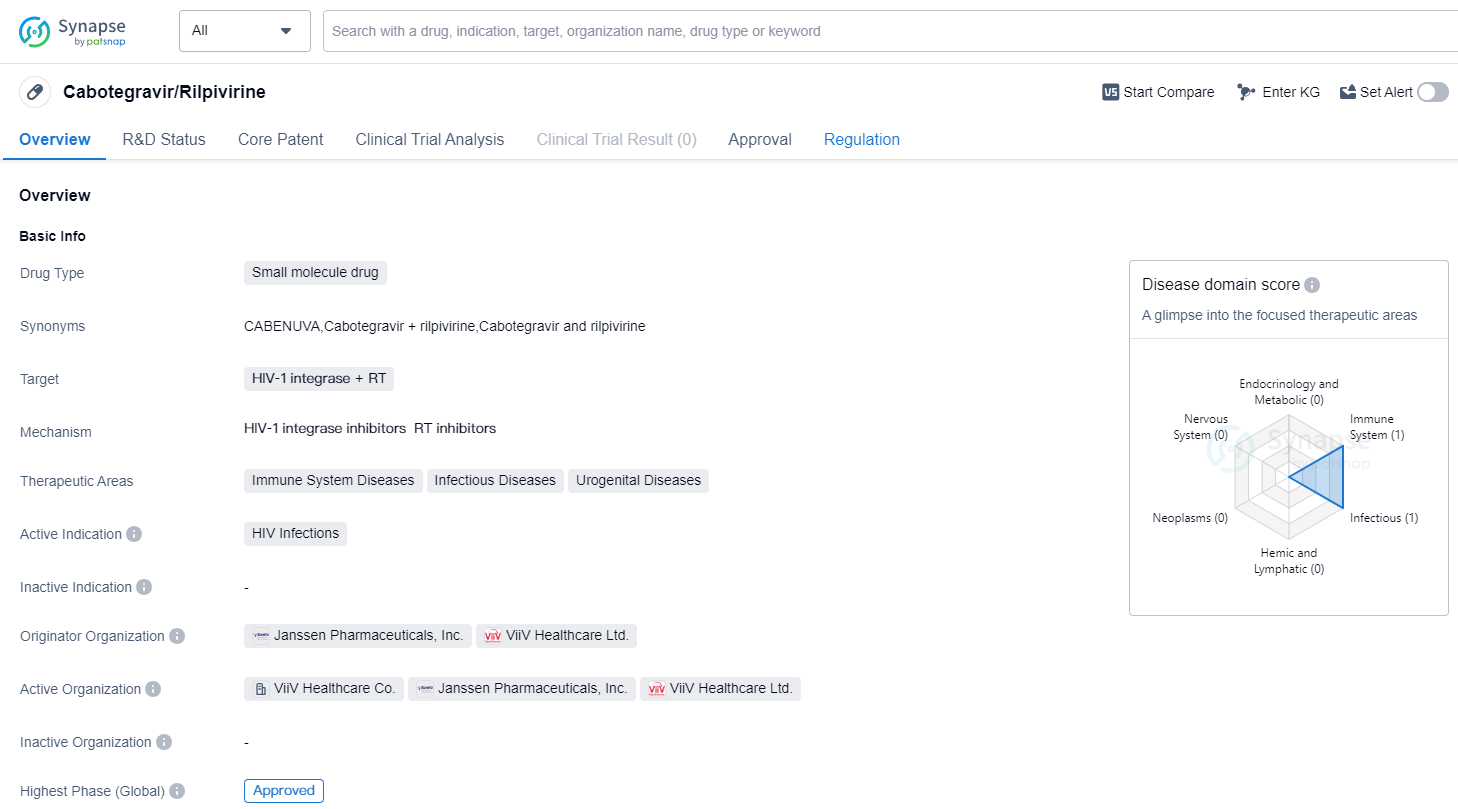
Cabotegravir/Rilpivirine is a small molecule drug that targets HIV-1 integrase and reverse transcriptase (RT). It falls under the therapeutic areas of immune system diseases, infectious diseases, and urogenital diseases, with its active indication being HIV infections. The drug was developed by Janssen Pharmaceuticals, Inc. and ViiV Healthcare Ltd., both reputable organizations in the pharmaceutical industry.
As of the highest phase, Cabotegravir/Rilpivirine has been approved globally. Its first approval was granted in March 2020 in Canada. The drug has undergone a fast track regulatory process, which indicates that it has been expedited due to its potential to address an unmet medical need or provide significant benefits over existing treatments.
Cabotegravir/Rilpivirine is a combination therapy that combines two active ingredients, cabotegravir and rilpivirine. Cabotegravir is an integrase inhibitor that works by blocking the action of the HIV-1 integrase enzyme, which is essential for the replication of the virus. Rilpivirine, on the other hand, is a non-nucleoside reverse transcriptase inhibitor that inhibits the reverse transcriptase enzyme, preventing the conversion of viral RNA into DNA.
The approval of Cabotegravir/Rilpivirine represents a significant advancement in the treatment of HIV infections. By targeting both the integrase and reverse transcriptase enzymes, the drug offers a dual mechanism of action, potentially enhancing its efficacy and reducing the risk of drug resistance.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for cabotegravir/rilpivirine: HIV-1 integrase inhibitors RT inhibitors
HIV-1 integrase inhibitors are a type of antiretroviral drug used in the treatment of HIV-1 infection. HIV-1 integrase is an enzyme that plays a crucial role in the replication of the virus. These inhibitors work by blocking the action of the integrase enzyme, preventing it from inserting the viral DNA into the DNA of the host cell. By inhibiting this step, the replication of the virus is halted, reducing the viral load in the body and slowing down the progression of the disease.
RT inhibitors, on the other hand, refer to reverse transcriptase inhibitors. Reverse transcriptase is another enzyme essential for the replication of HIV-1. These inhibitors target the reverse transcriptase enzyme, preventing it from converting the viral RNA into DNA. By inhibiting reverse transcriptase, these drugs interfere with the replication process of the virus, ultimately reducing the viral load and slowing down the progression of HIV-1 infection.
Both HIV-1 integrase inhibitors and RT inhibitors are important components of combination antiretroviral therapy (cART) used to manage HIV-1 infection. They are typically used in combination with other antiretroviral drugs to maximize their effectiveness and minimize the development of drug resistance.
Drug Target R&D Trends for cabotegravir/rilpivirine
HIV-1 integrase + RT (reverse transcriptase) plays a crucial role in the human body during HIV infection. Integrase is an enzyme responsible for integrating the viral DNA into the host cell's genome, allowing the virus to replicate and persist. RT, on the other hand, is an enzyme that converts the viral RNA into DNA, a critical step in the replication process. Together, integrase and RT facilitate the establishment and maintenance of HIV infection within the human body. Understanding the mechanisms and functions of these enzymes is essential for developing effective antiretroviral therapies to combat HIV/AIDS.
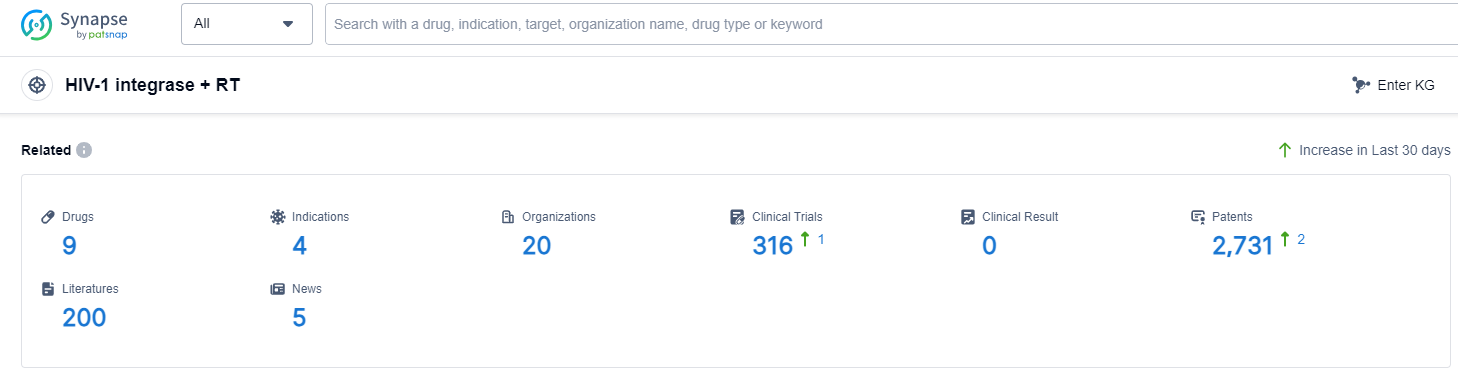
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 9 HIV-1 integrase + RT drugs worldwide, from 20 organizations, covering 4 indications, and conducting 316 clinical trials.
The analysis of the current competitive landscape of target HIV-1 integrase + RT reveals that GSK Plc and Gilead Sciences, Inc. are the leading companies in terms of R&D progress. The main indications for the approved drugs under this target are HIV Infections and HIV-1 infection. Small molecule drugs are the most rapidly progressing drug type, indicating intense competition in this field. The United States, European Union, and Canada are the leading countries/locations in terms of drug development, with China also showing progress. Overall, the target HIV-1 integrase + RT has a competitive landscape with multiple companies, indications, drug types, and countries/locations involved in its development. The future development of this target holds promise for the advancement of HIV treatment options.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Cabotegravir/Rilpivirine is a small molecule drug developed by Janssen Pharmaceuticals, Inc. and ViiV Healthcare Ltd. It has been approved globally for the treatment of HIV infections and offers a combination therapy approach targeting HIV-1 integrase and reverse transcriptase. The drug's fast-track regulatory status highlights its potential to benefit patients by addressing unmet medical needs.