Exploring CC-91633: A Novel Molecular Glue Drug Targeting CK1α for Cancer and Blood Disorders
CC-91633 is a novel molecular glue drug that targets CK1α and falls within the therapeutic areas of neoplasms, hemic and lymphatic diseases. The drug's active indications include myelodysplastic syndromes, relapsing acute myeloid leukemia, and acute myeloid leukemia. It is developed by Celgene Corp., which is the originator organization of this drug. Currently, the drug is in its highest global phase, which is Phase 1.
Molecular glue drugs are a new class of small molecules that induce protein degradation. The specific targeting of CK1α suggests that CC-91633 may work by leveraging the molecular glue approach to induce the degradation of specific disease-causing proteins associated with the indicated conditions. The therapeutic areas of neoplasms, hemic and lymphatic diseases indicate that this drug could potentially be used to treat various types of cancers and blood disorders.
The active indications of myelodysplastic syndromes, relapsing acute myeloid leukemia, and acute myeloid leukemia indicate that CC-91633 is being developed specifically for these conditions. These are serious and life-threatening diseases, therefore the development of a novel drug like CC-91633 could potentially address unmet medical needs and provide new treatment options for patients with these conditions.
Below, we will use the drug CC-91633 as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
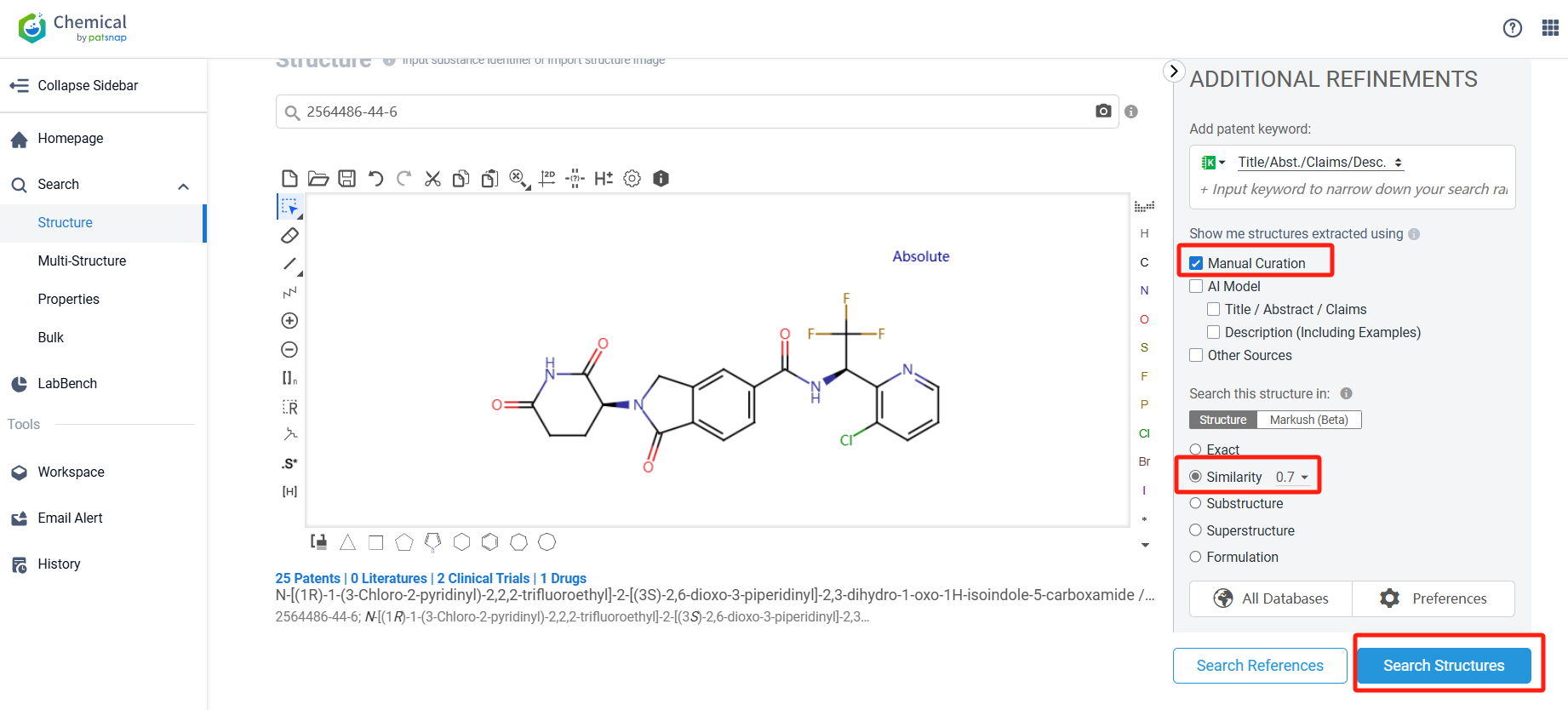
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of CC-91633 (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, using a similarity search (setting the Tanimoto coefficient to 0.7), check the box for manual curation, click on search structures, and you can find the innovative drug CC-91633, as disclosed in the patent application with the publication number WO2020243379A1, first made public on 2020-12-03.
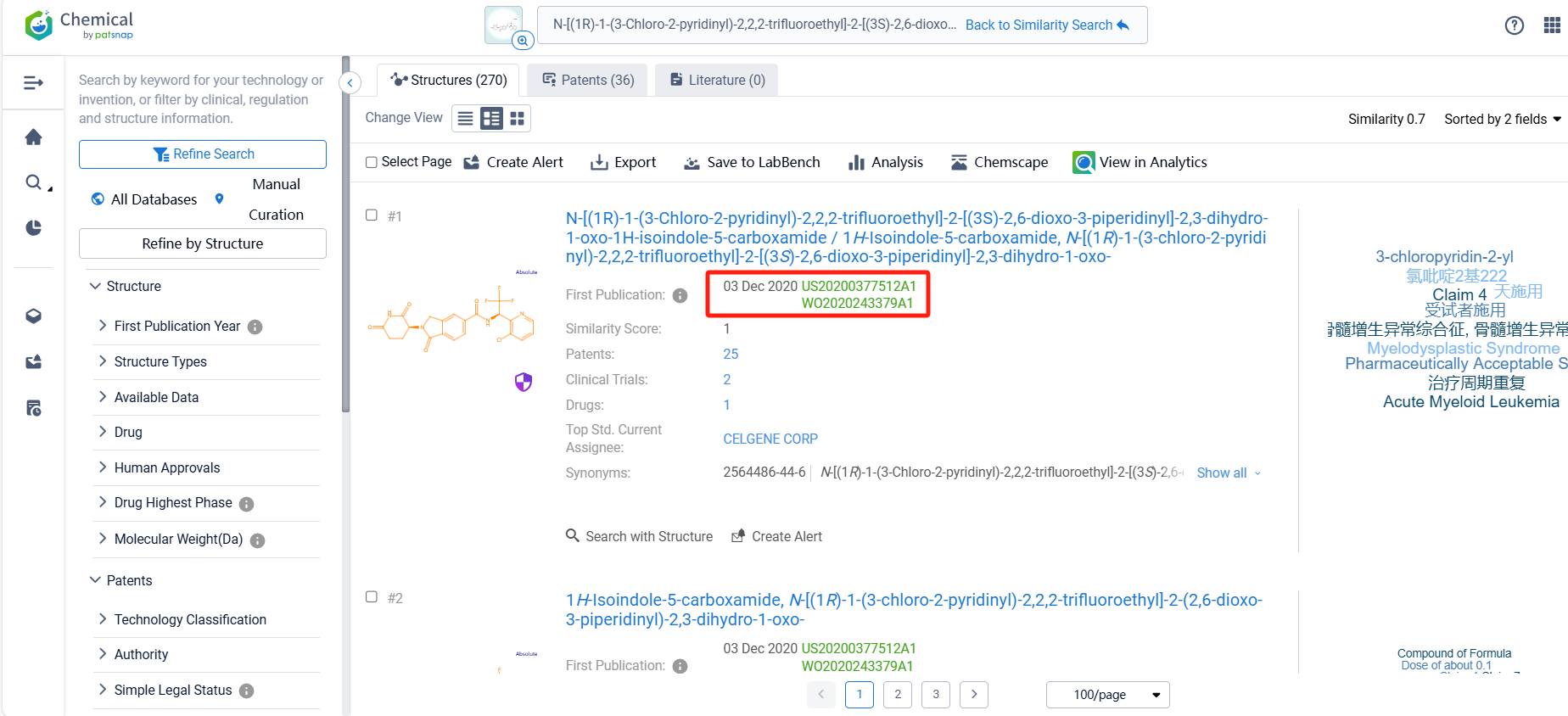
There are 36 patents related to this compound. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
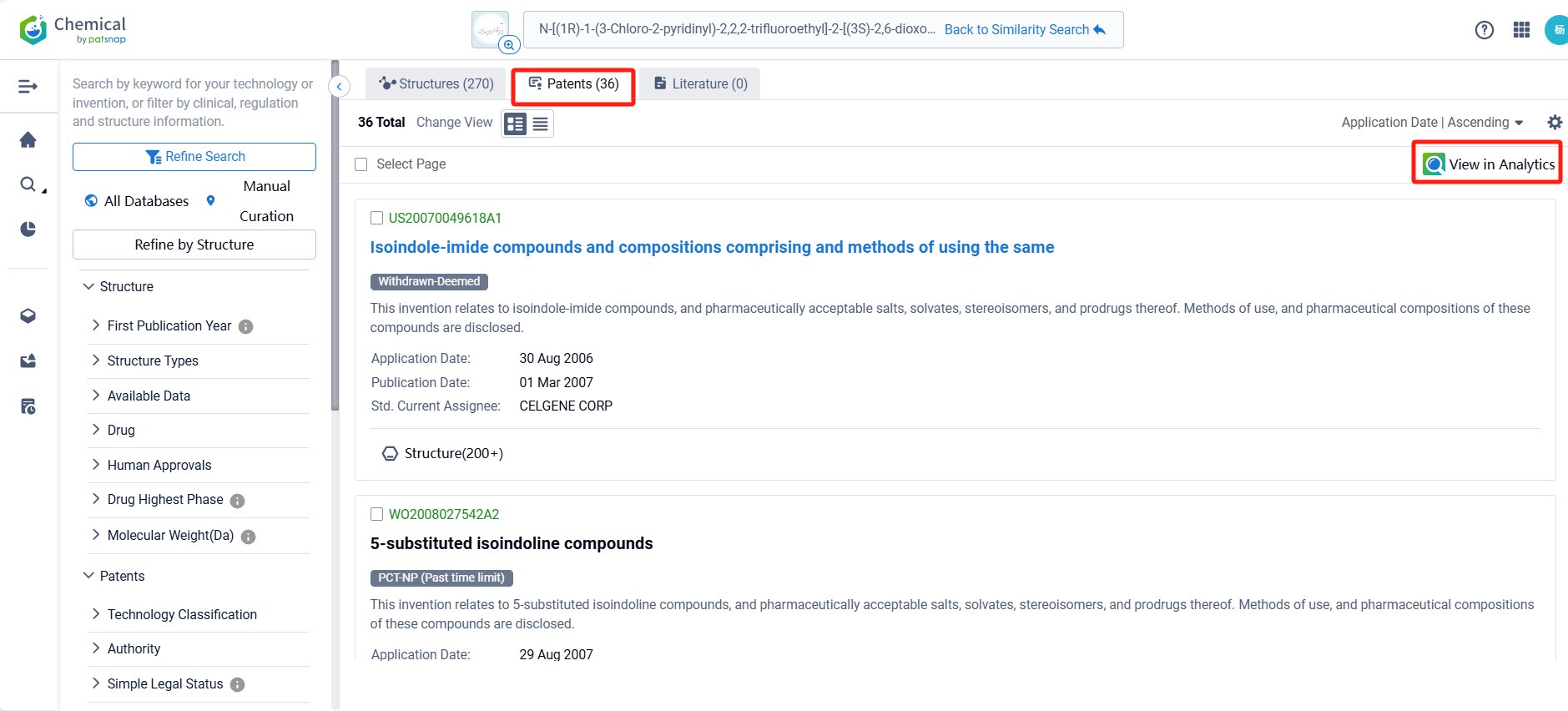
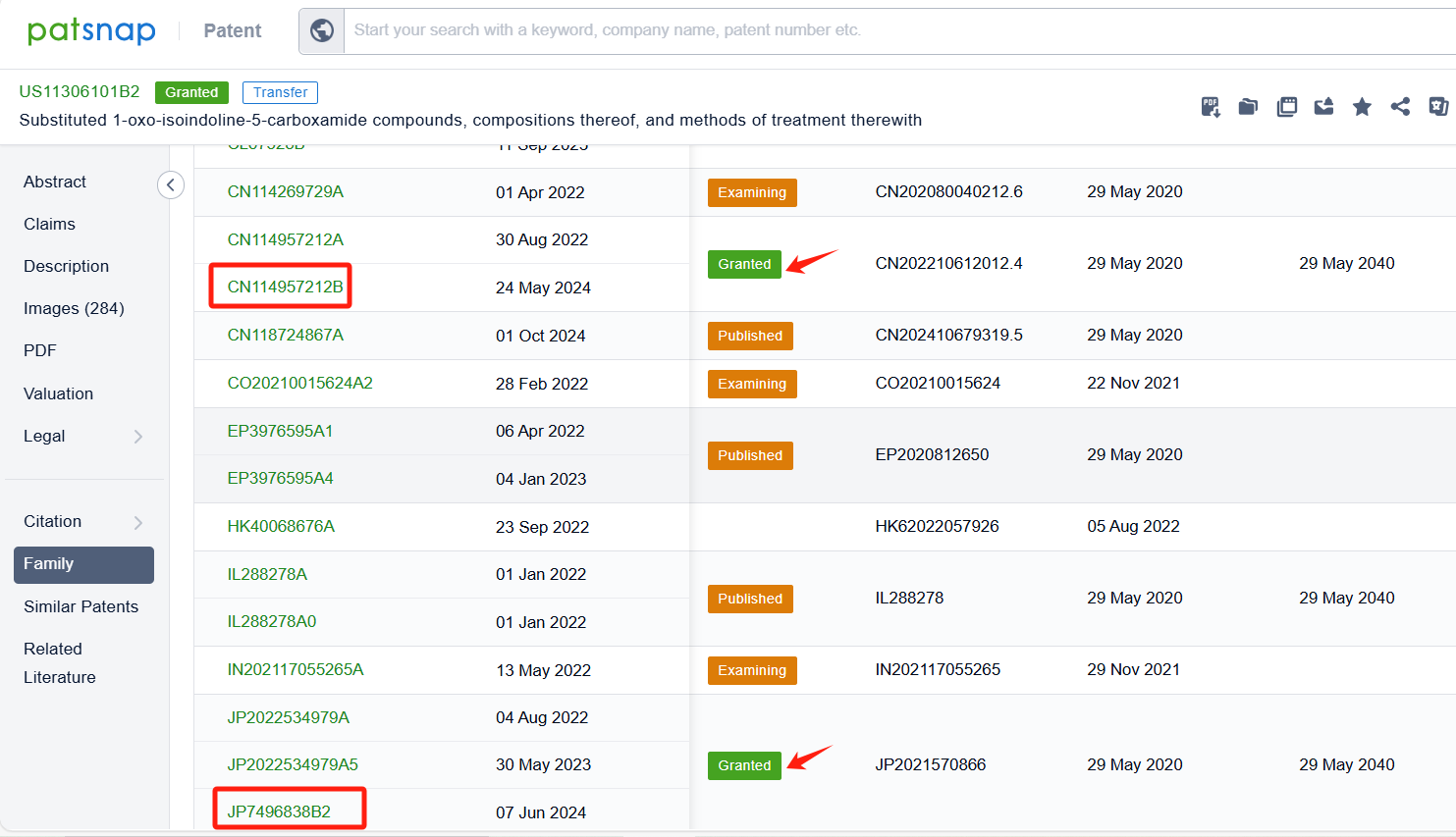
By reviewing the aforementioned patents, we can observe that the core United States patent related to this compound has been granted, with the grant publication number US11306101B2, the grant date being April 19, 2022, and the estimated expiration date May 29, 2040. I The Chinese and Japanese counterparts of the compound have also been granted, with the grant publication numbers CN114957212B and JP7496838B2, respectively.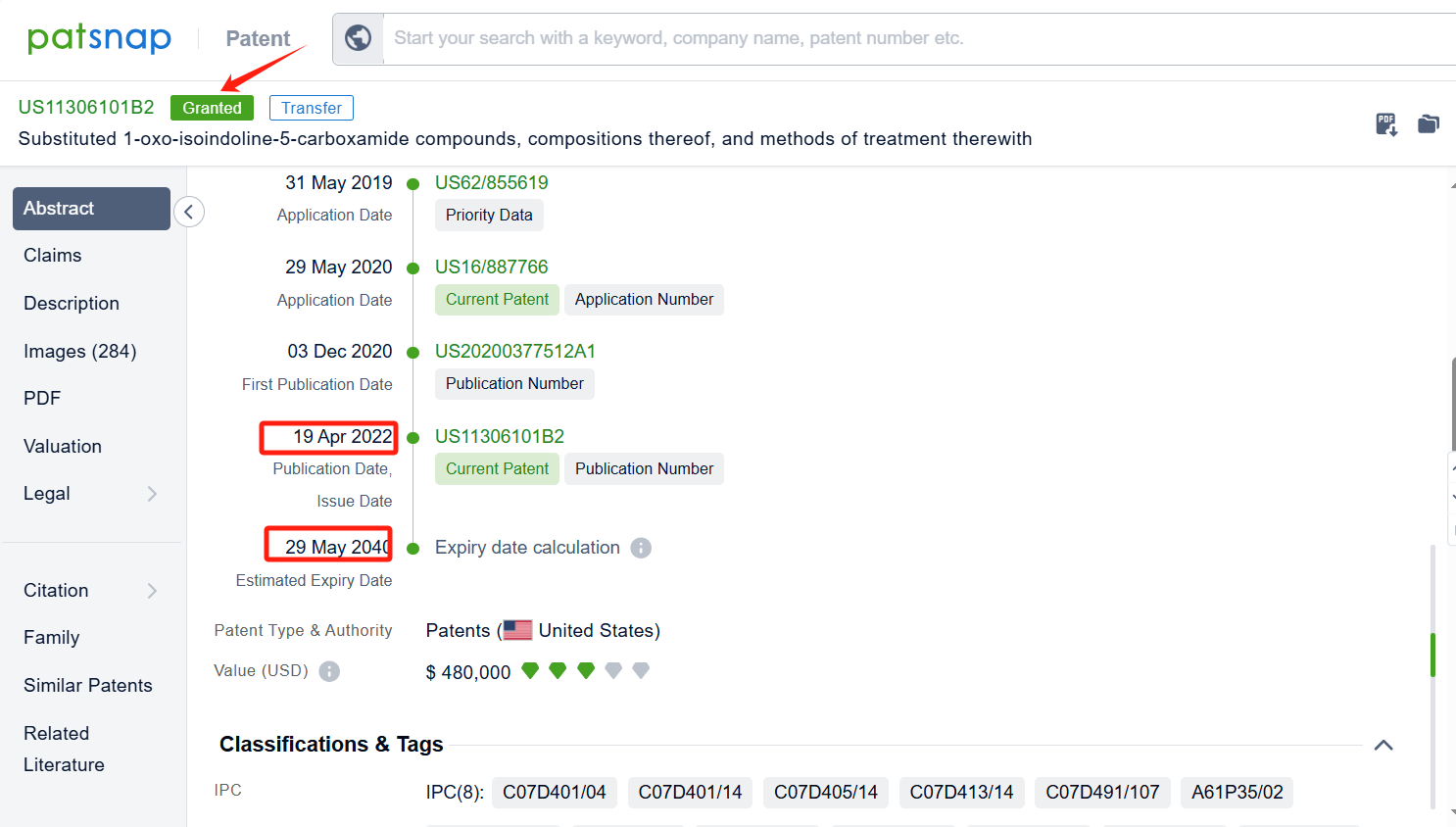
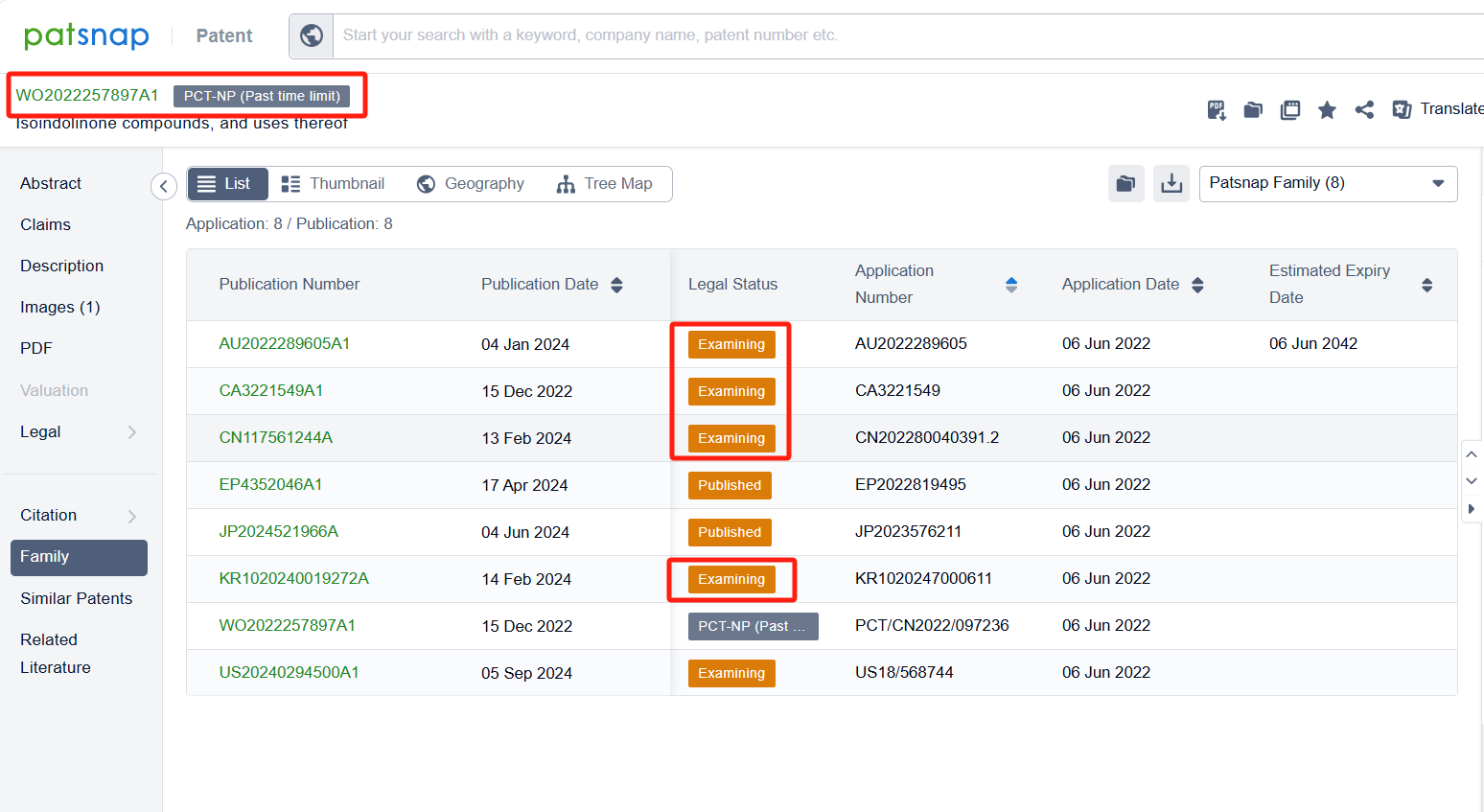
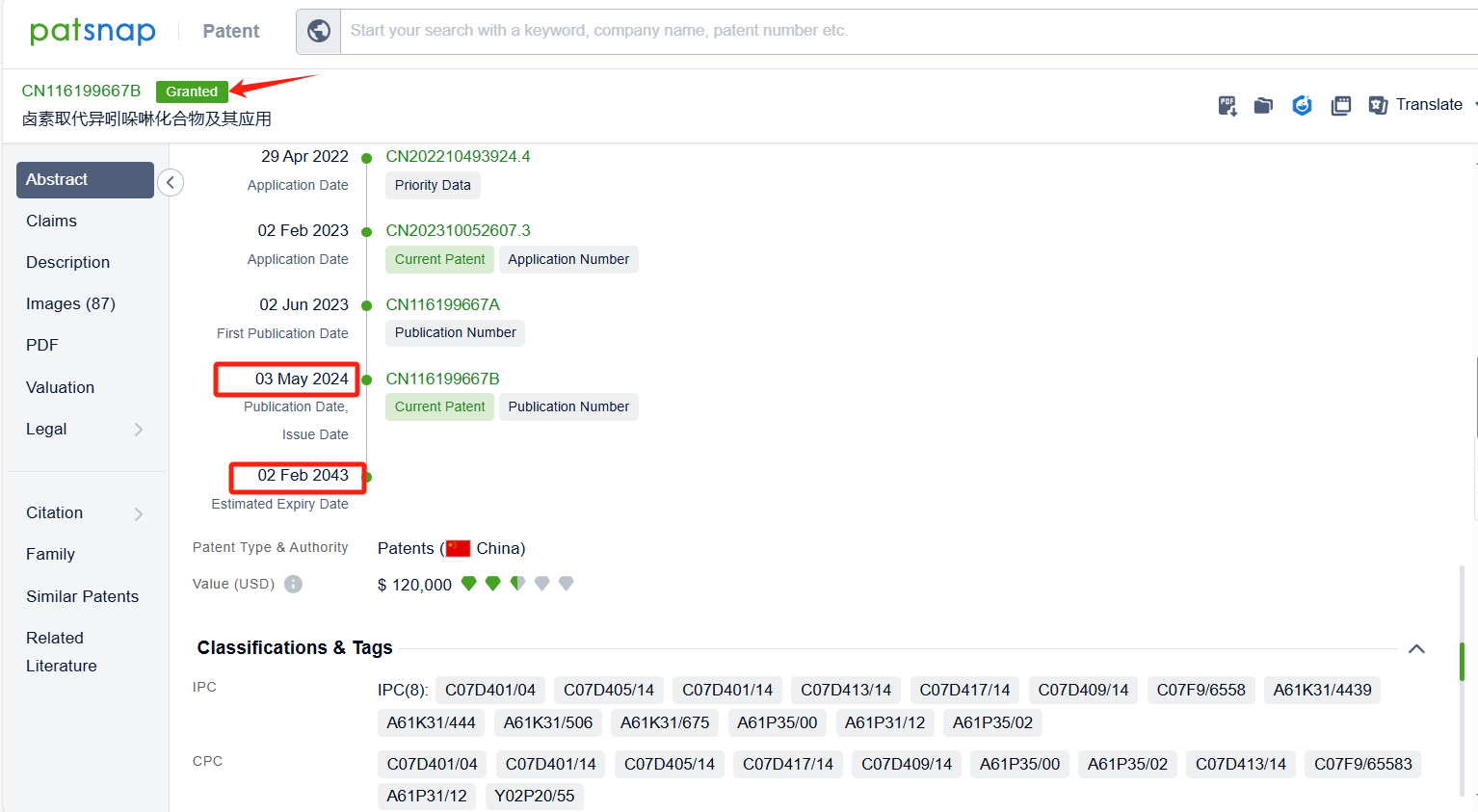
Among the applicants of the patent, one can find other companies' fast follow patents on Celgene Corp.; for example, Hangzhou Glubio Pharmaceutical CO., LTD.'s international PCT application (WO2022257897A1) has entered the national phase in designated countries and is undergoing substantive examination in countries such as China, South Korea, and Australia. Chengdu FenDi Pharmaceutical Co., Ltd.'s patent application patent application CN116199667B has also been granted, with a grant date of 2024-05-03, and an estimated expiration date of 2042-02-02.
As the highest global phase for CC-91633 is Phase 1, this suggests that the drug is currently undergoing initial clinical trials to evaluate its safety, dosage, and potential efficacy in human patients. Given the early stage of development, further clinical trials will be required to assess the drug's overall safety and efficacy profile before potential regulatory approval and commercialization.
In summary, CC-91633 is a novel molecular glue drug targeting CK1α with active indications in myelodysplastic syndromes and various forms of acute myeloid leukemia. Developed by Celgene Corp., the drug is currently in Phase 1 of its development, indicating it is in the early stages of clinical evaluation. The potential for CC-91633 to provide new treatment options for these serious and life-threatening conditions makes it a drug of significant interest within the biomedical and pharmaceutical industry.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.