Exploring DKN-01's R&D successes and its clinical results at the 2024 ASCO_GI
Colorectal adenocarcinoma (CRC) is characterized by hyperactivation of the Wnt pathway where DKK1 plays a critical regulatory role. DKN-01 is an IgG4 monoclonal antibody that potently neutralizes DKK1. On 18 Jan 2024, the latest clinical data of DKN-01 for the treatment of CRC were presented in 2024 ASCO_GI.
DKN-01's R&D Progress
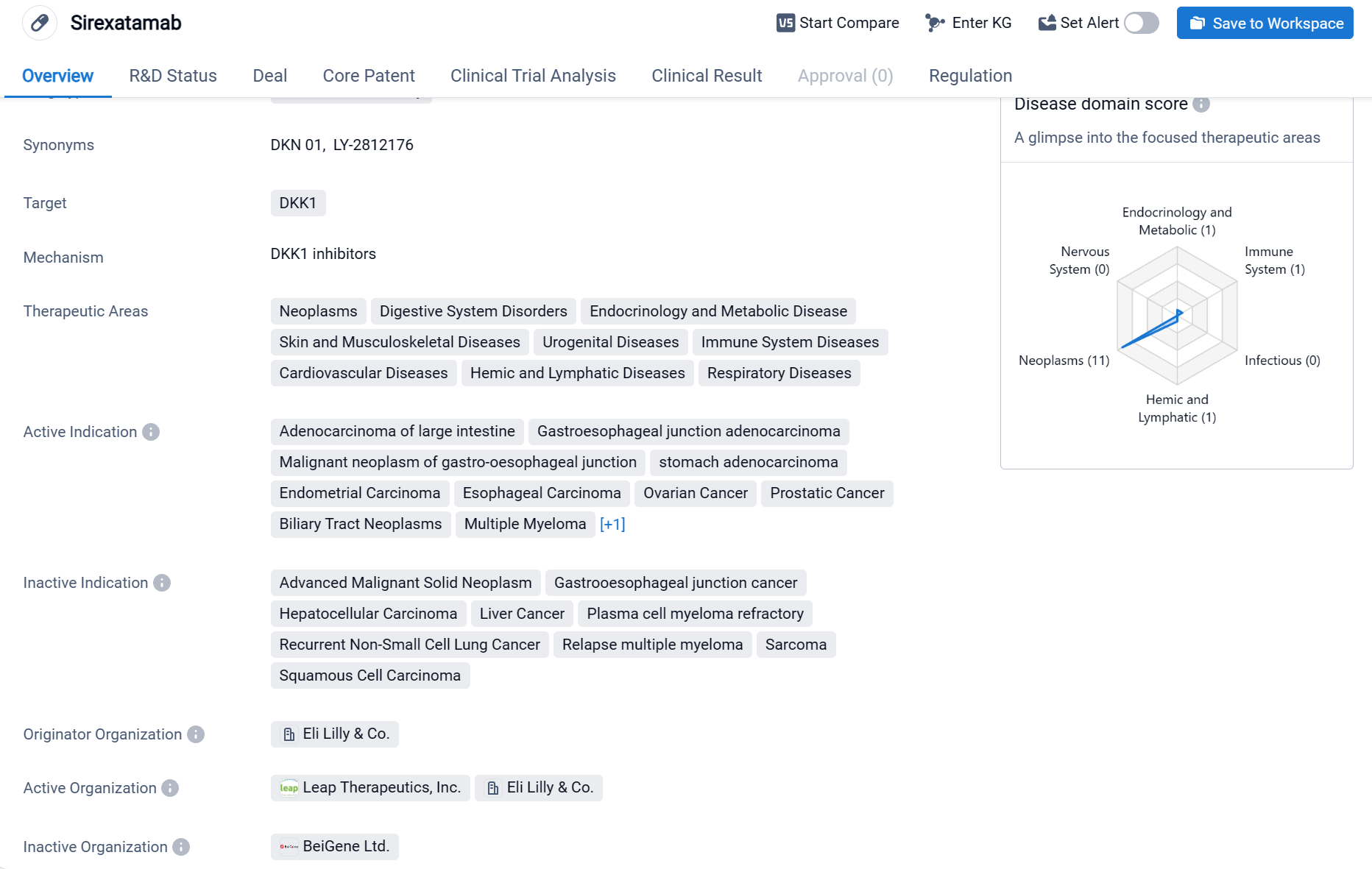
DKN-01 is a monoclonal antibody drug developed by Eli Lilly & Co. It falls under the therapeutic area of biomedicine and is primarily targeted towards the treatment of various neoplasms, digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, urogenital diseases, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, and respiratory diseases.
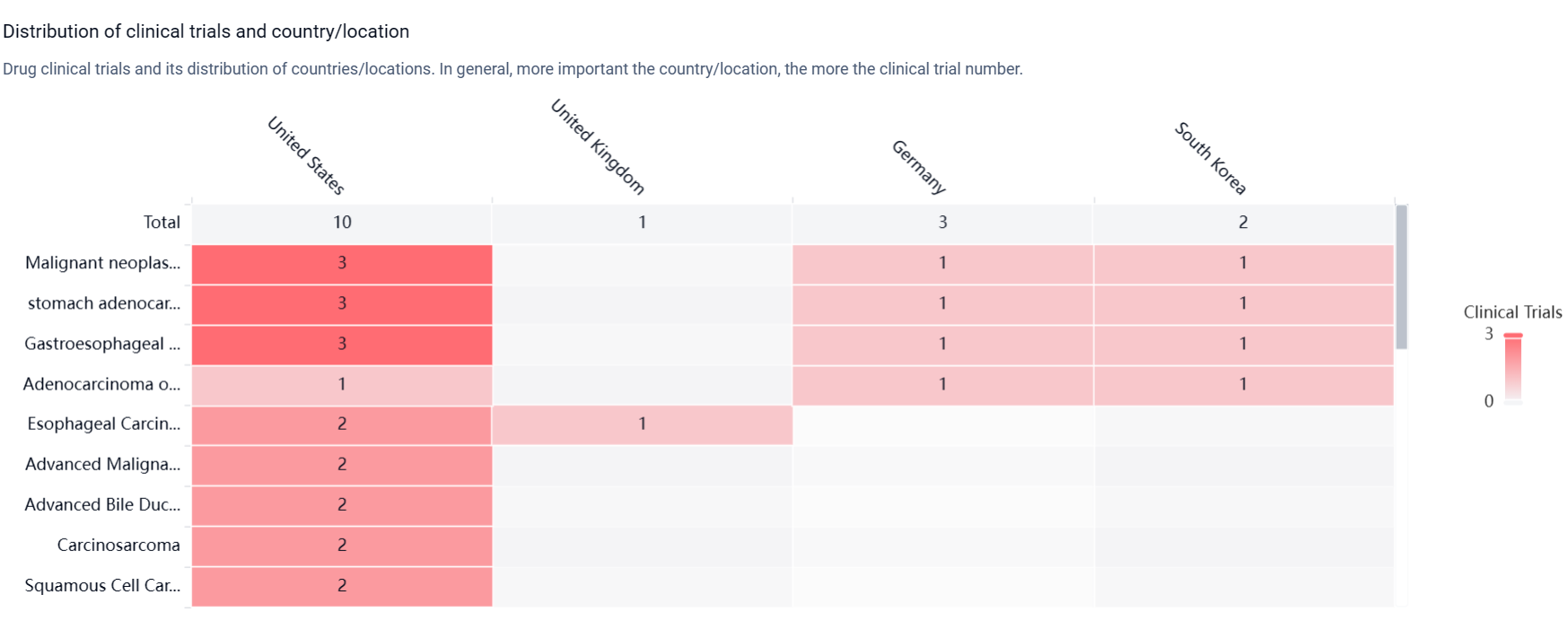
According to the Patsnap Synapse, DKN-01 is in the highest phase of clinical development, which is Phase 2. And the clinical trial distributions for DKN-01 are primarily in the United States, United Kingdom and Germany. The key indication is Malignant neoplasm of gastro-oesophageal junction.
Detailed Clinical Result of DKN-01
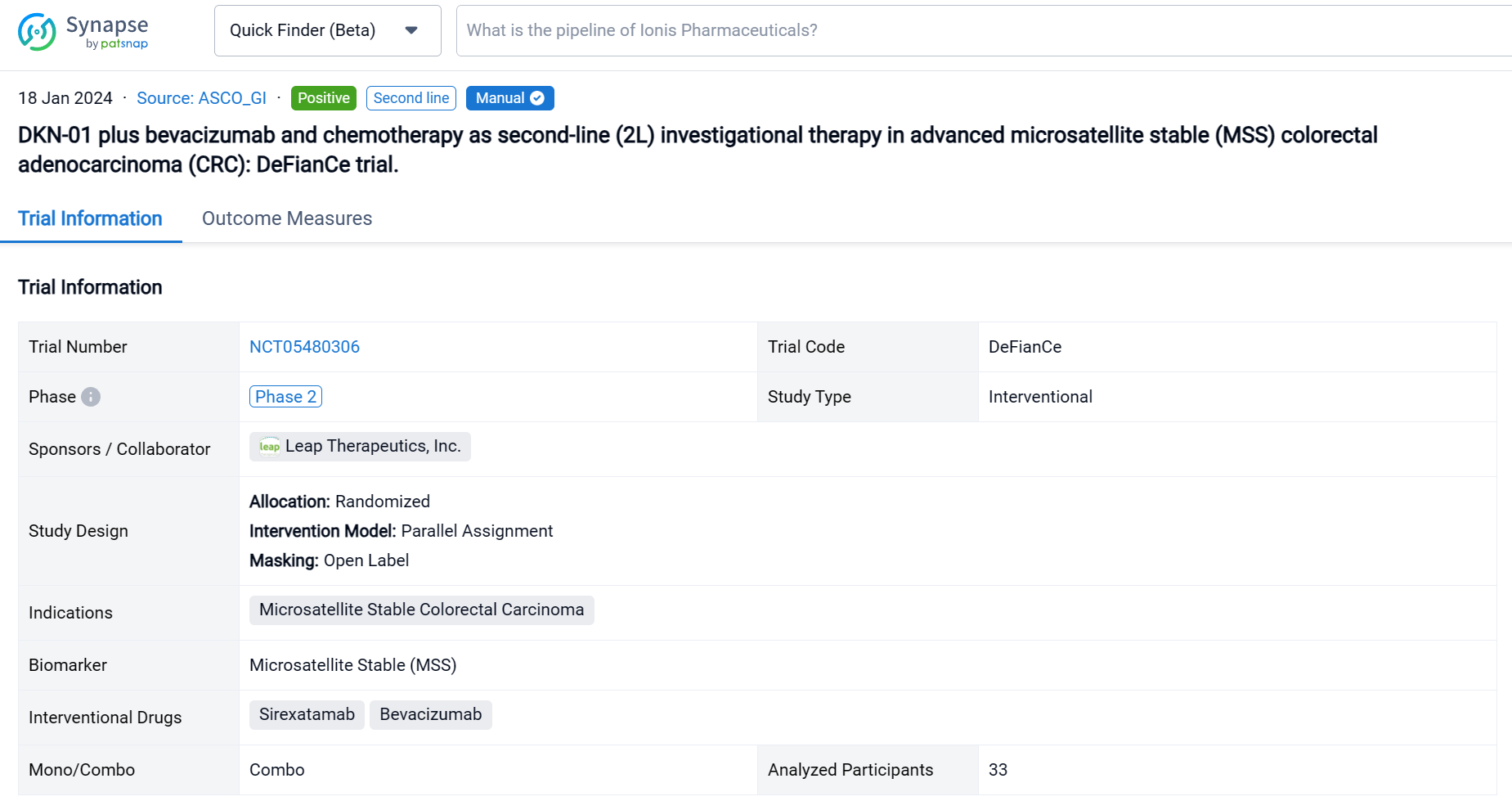
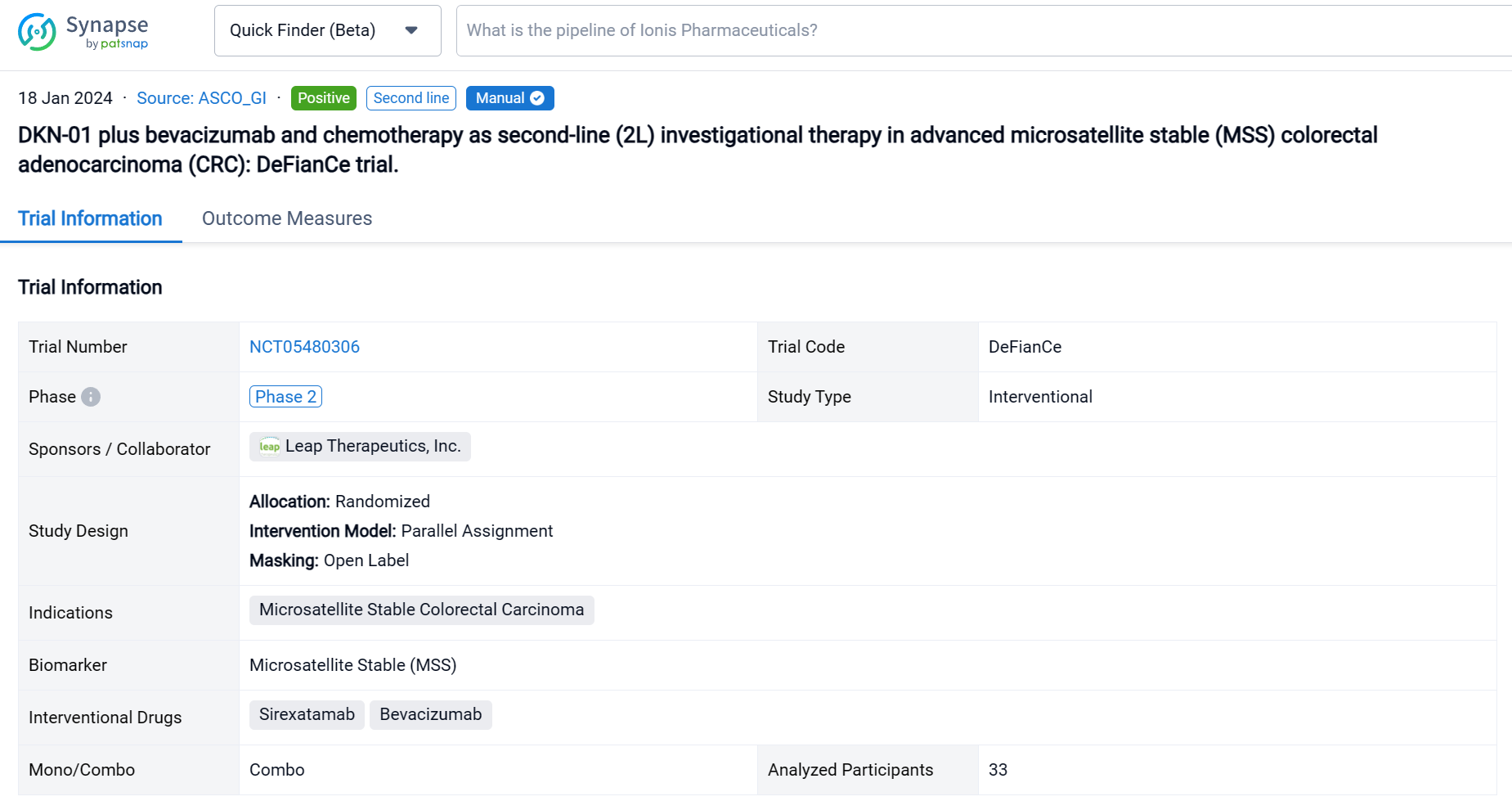
This phase 2 randomized, open-label, two-part, multicenter study (NCT05480306) was aimed to evaluate efficacy and safety of DKN-01 plus FOLFIRI/FOLFOX and bevacizumab versus standard of care (SOC) [FOLFIRI/FOLFOX and bevacizumab] as 2L treatment of advanced MSS CRC patients (pts).
In this study, the primary endpoint of the single arm Part A (DKN-01 + SOC) was safety and tolerability with secondary endpoints including overall response rate (ORR), progression-free survival (PFS) and overall survival. Retrospective analysis of tumoral DKK1 mRNA expression was measured centrally by in situ hybridization.
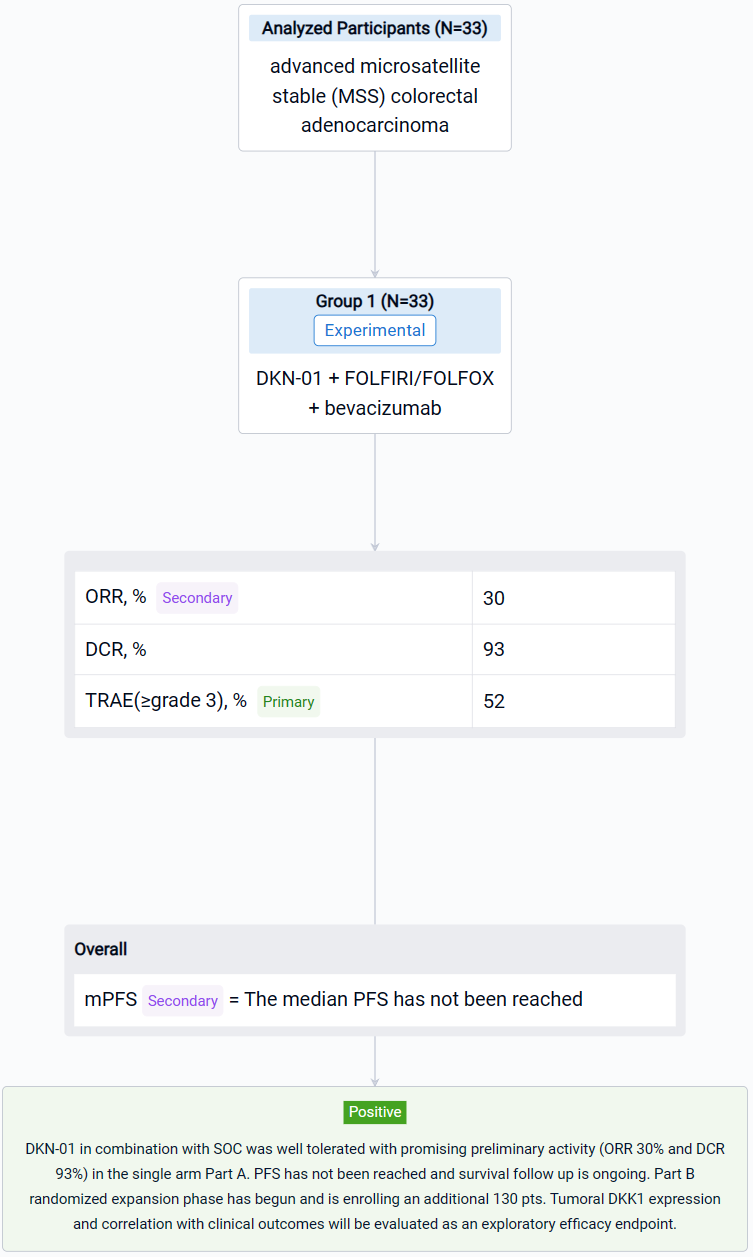
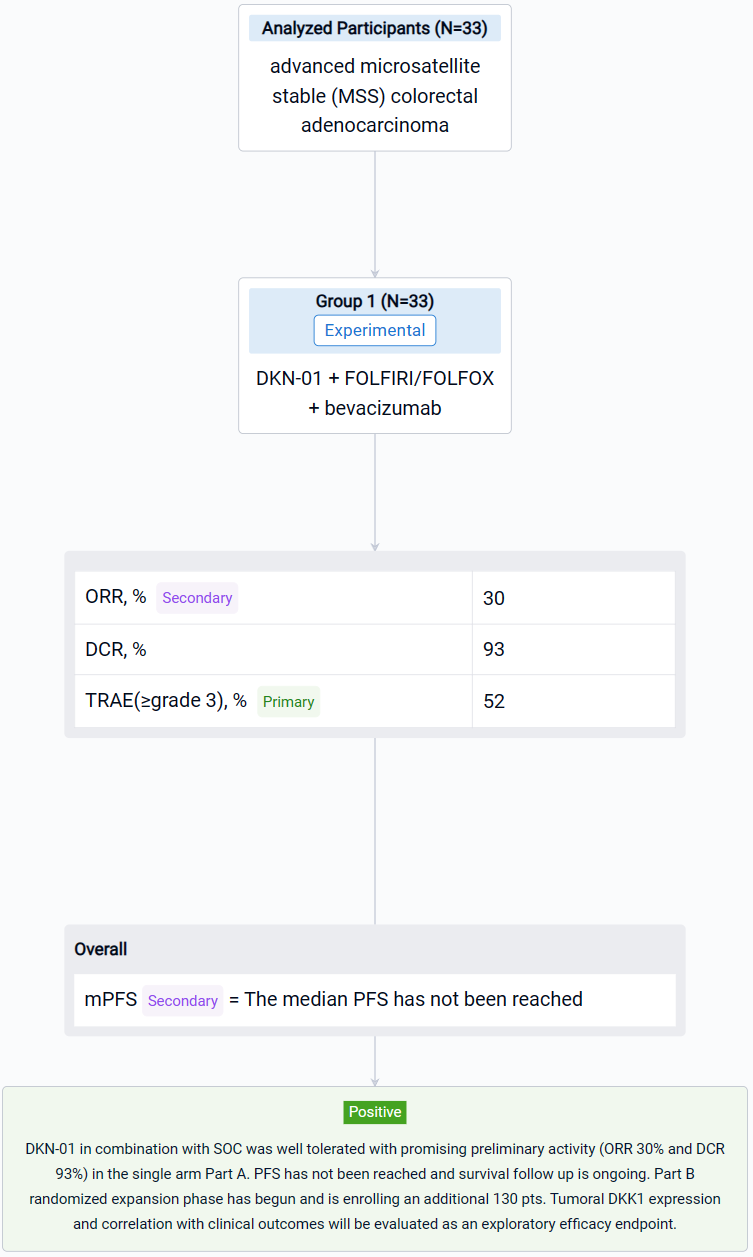
The result showed that Thirty-three pts enrolled in Part A between Sept 2022-April 2023. As of 21 Aug 2023, median age 56 years (35, 84); 20 males. 29 pts had tumors with evaluable baseline DKK1 expression; 55% were DKK1-expressing (≥1% tumor cells). All patients had received prior 5FU-based therapies, 30 pts (91%) with oxaliplatin in combination and 17 pts (52%) had prior bevacizumab. 24 pts (73%) had RAS mutations (22 KRAS, 2 NRAS) and 23 pts (70%) had liver metastasis. 30 pts (91%) received DKN-01 + bevacizumab + FOLFIRI. 17 pts (52%) had ≥grade 3 treatment related adverse events (TRAE) with neutrophil count decreased, anemia and fatigue representing the most common. No pt discontinued DKN-01 due to a TRAE. Of the 27 response evaluable pts the ORR was 30% and the DCR was 93%: 8 PR, 17 SD and 2 PD. The median PFS has not been reached.
It can be concluded thatDKN-01 in combination with SOC was well tolerated with promising preliminary activity (ORR 30% and DCR 93%) in the single arm Part A. PFS has not been reached and survival follow up is ongoing. Part B randomized expansion phase has begun and is enrolling an additional 130 pts. Tumoral DKK1 expression and correlation with clinical outcomes will be evaluated as an exploratory efficacy endpoint.
How to Easily View the Clinical Results Using Synapse Database?
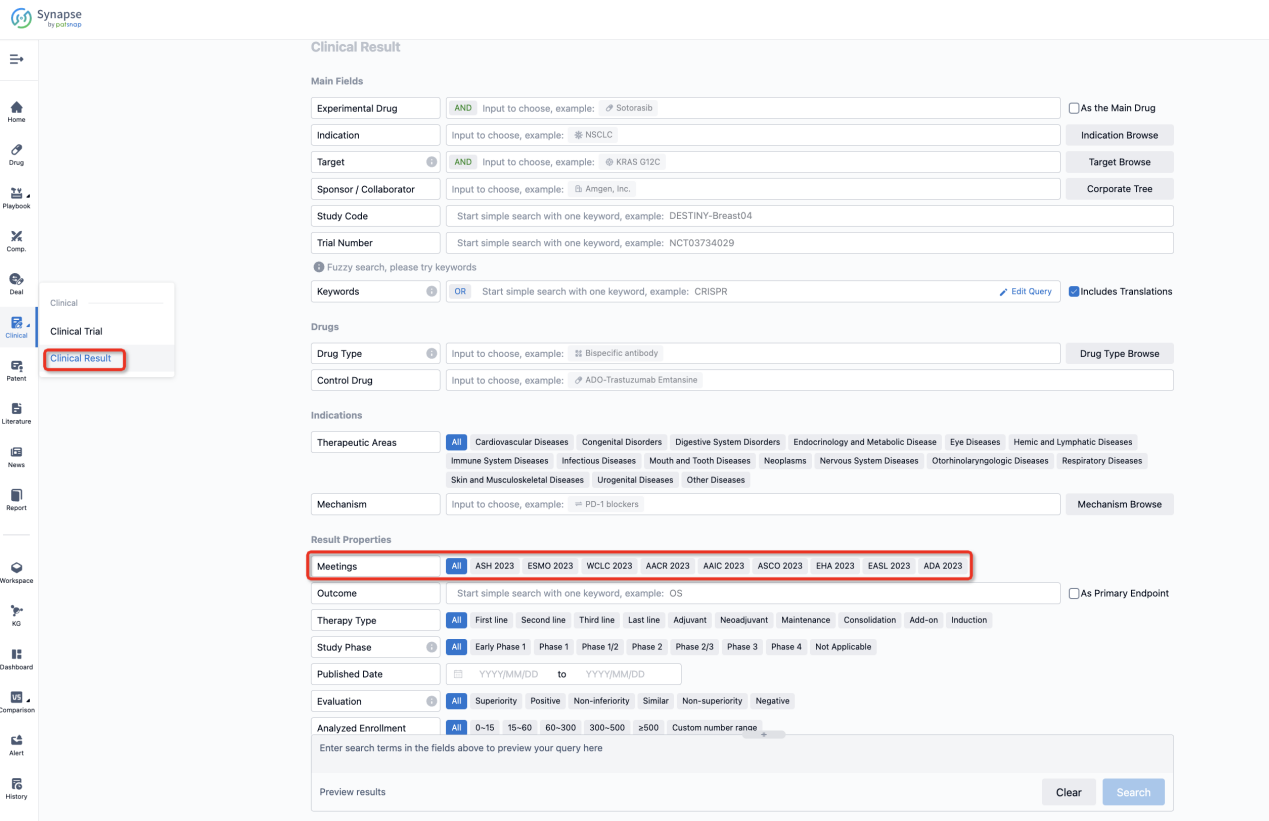
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
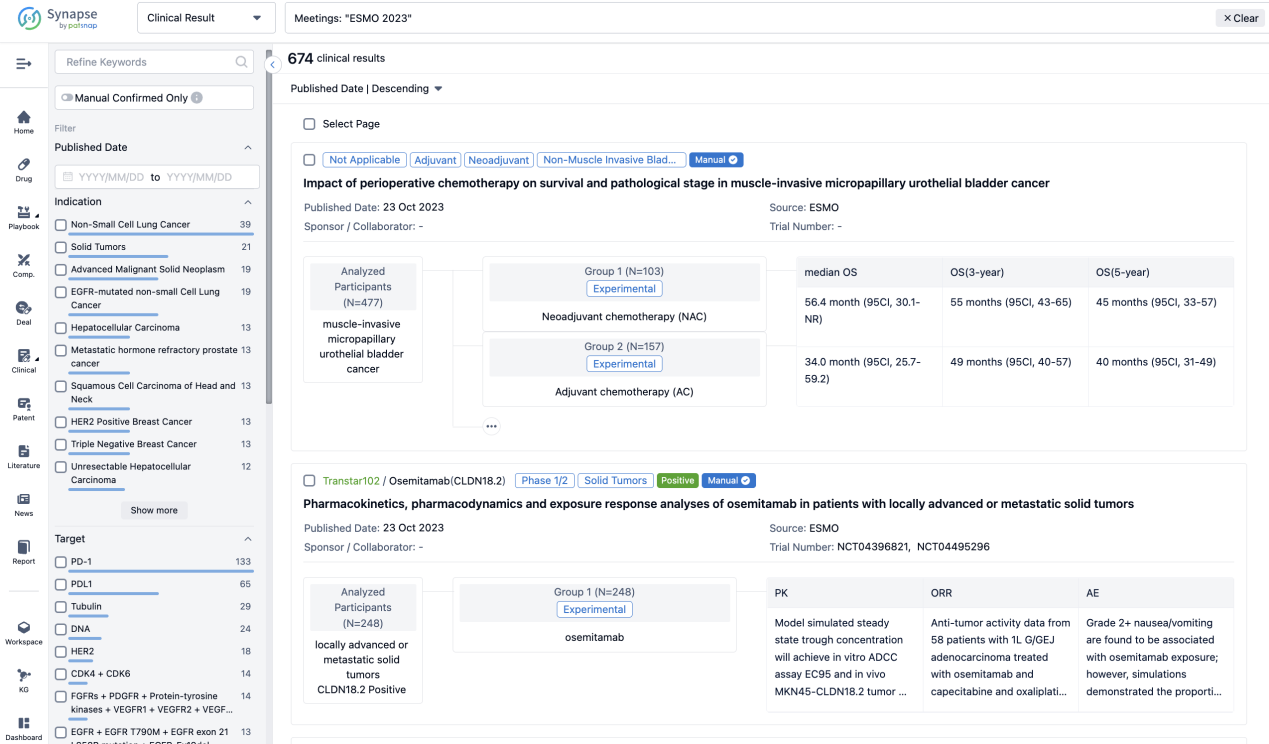
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
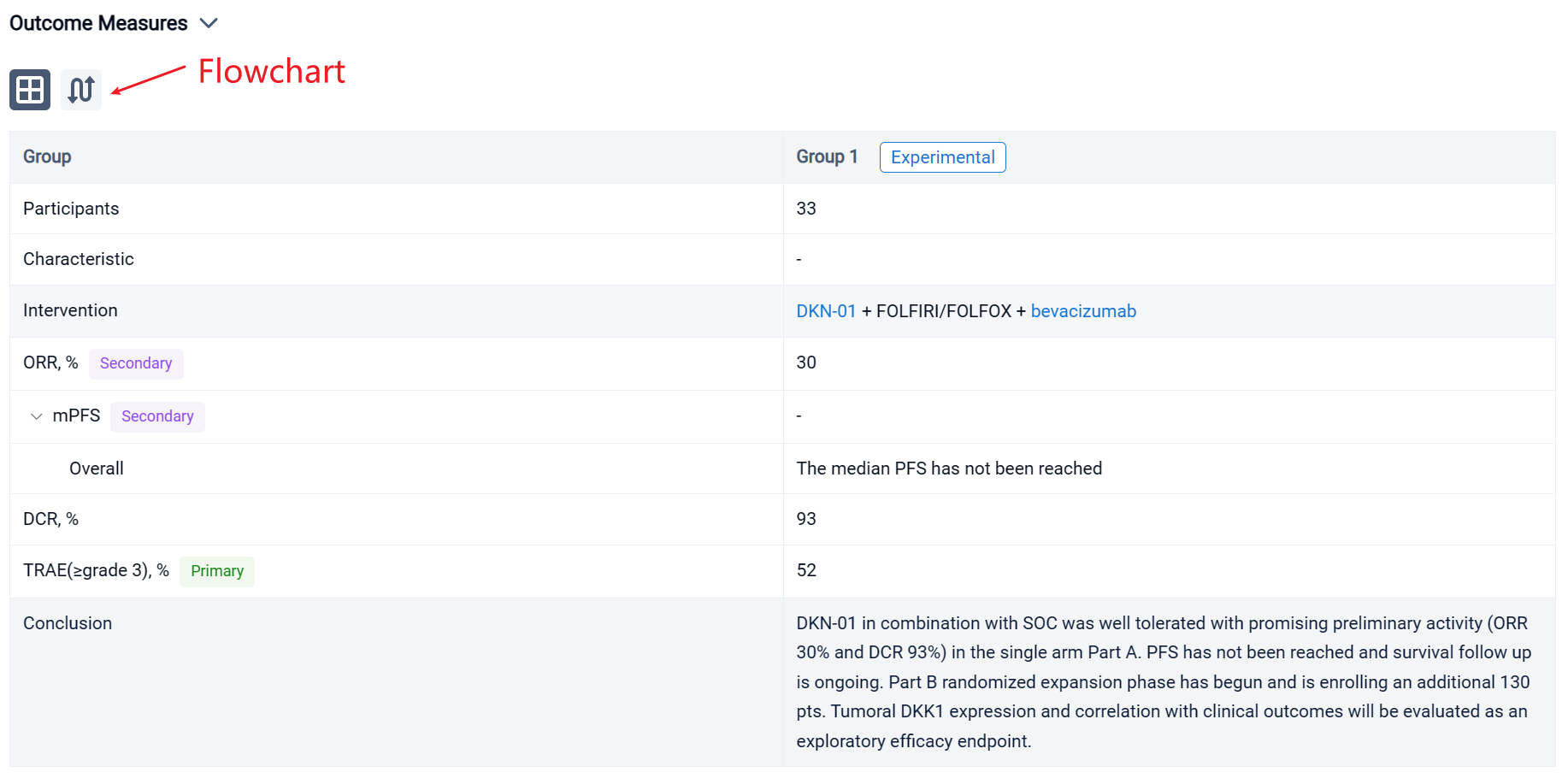
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
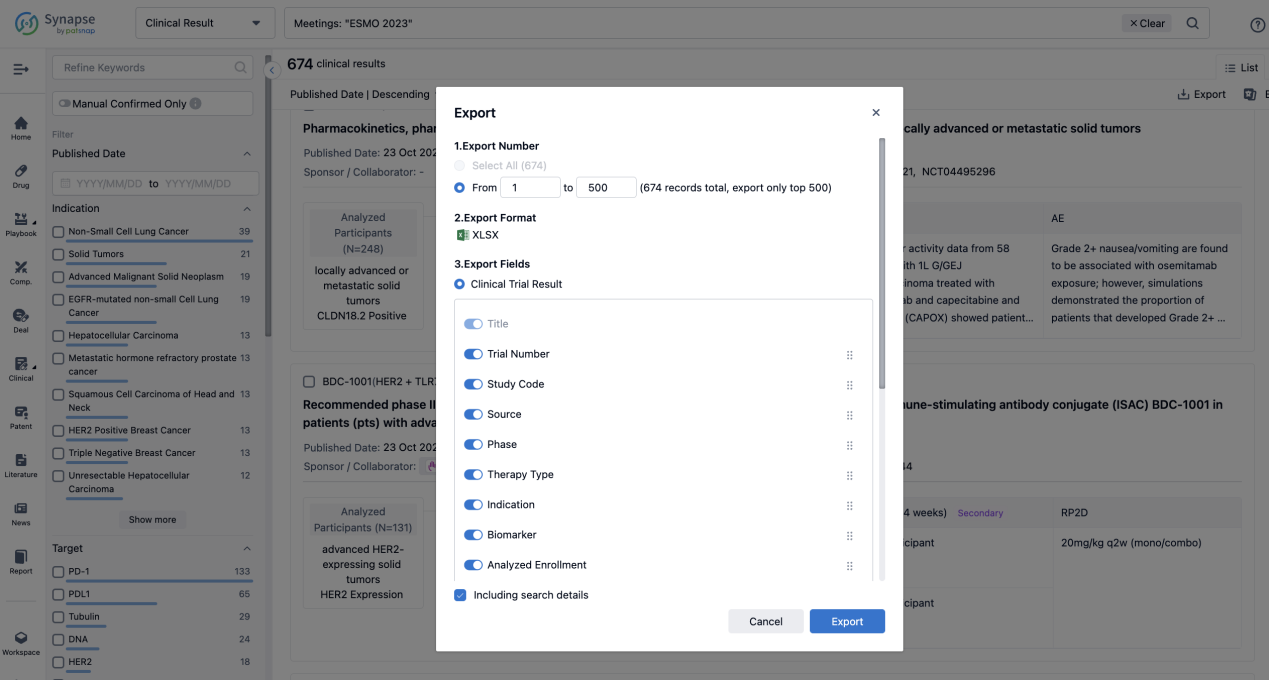
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!