Technoderma's TDM-105795 Stage 2 Trial Shows Positive Hair Regrowth in Pattern Baldness
It is with great pride that we announce Technoderma Medicines, Inc., has successfully concluded the Phase 2a trials for its innovative topical treatment, TDM-105795, which targets Androgenetic Alopecia. Our firm, situated in the avant-garde field of biopharmaceuticals and currently navigating through the clinical development stages, is excited about the implications of this accomplishment for the Company's future endeavors.
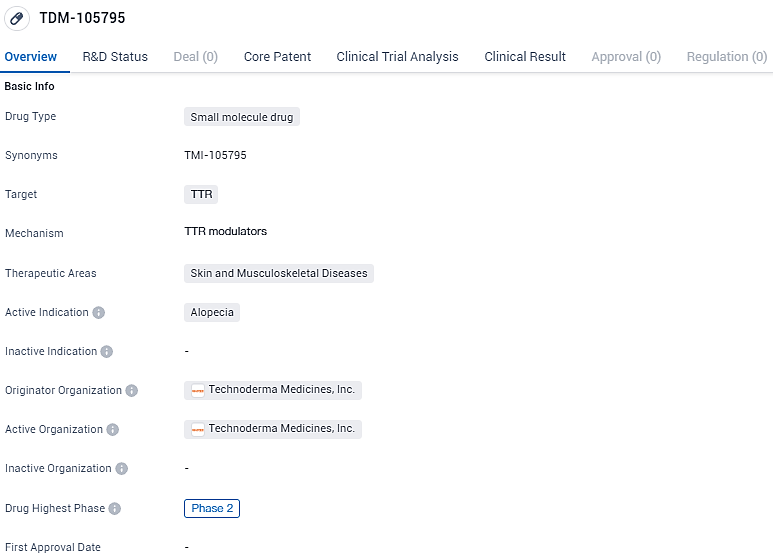
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In this pilot clinical study conducted within the AGA initiative, participants received a four-month regimen with a focus on the administration of a daily dose. The trial was labled as "A Randomized, Double-Blind, Vehicle-Controlled, Parallel Group, Multi-Dose Study to Evaluate the Efficacy and Safety of TDM-105795 in Male Subjects with Androgenetic Alopecia."
The primary aims of the trial were the assessment of early-stage effectiveness, safety, and drug absorption levels for TDM-105795 used topically; this study saw the collaboration of thirteen clinical centers across the United States, all operating under an open Investigational New Drug (IND) application with the FDA. The effectiveness was quantified by tracking the non-vellus Target Area Hair Count, revealing an average increase from the initial values of 24.3 hairs for the high concentration formulation and 20.3 hairs for the low concentration topical solution, in contrast to the 14.0 hairs observed with the placebo over areas measuring 1 cm2. The study had a 1:1:1 randomized allocation with 71 participants.
TDM-105795 demonstrated good tolerance at both concentrations without substantial safety concerns being noted. The bioanalytical data indicated that using the TDM-105795 topical solution once daily at either tested strength resulted in minimal to undetectable levels of systemic absorption.
The Chief Medical Officer at Technoderma Medicines, Arthur P. Bertolino, MD, PhD, MBA, expressed optimism based on Phase 2 outcomes suggesting that TDM-105795 incited hair growth associated with AGA, all the while maintaining a very promising safety record. The team anticipates further progressive steps in the clinical evaluations to discern the effects of prolonged treatment on overall hair density enhancement.
Technoderma Medicines' Chief Executive Officer, Zengquan Wang, PhD, emphasized this achievement as a significant verification of the company's potential to generate and devise new medicinal compounds within the dermatological sphere. The commitment remains strong towards cultivating a broad spectrum of cutting-edge drugs for dermatological conditions.
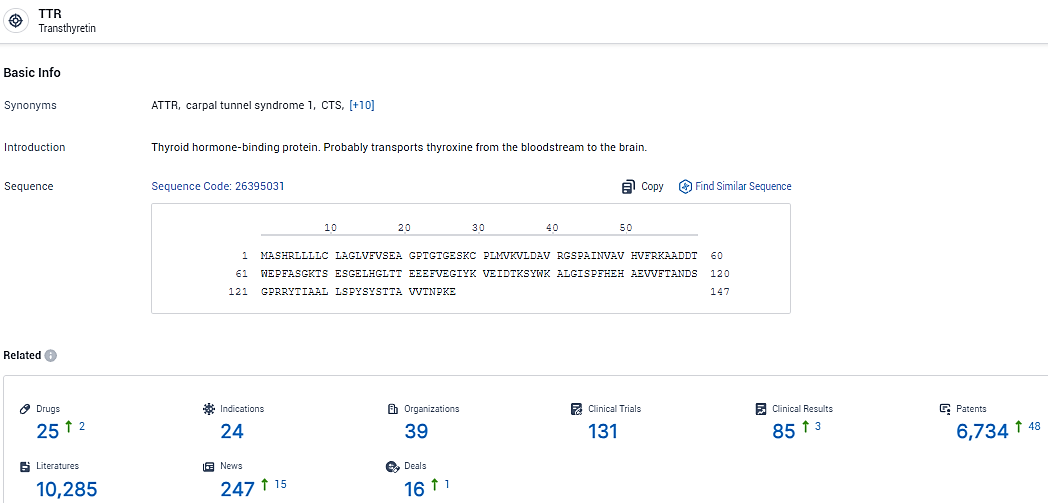
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 18, 2024, there are 25 investigational drugs for the TTR target, including 24 indications, 39 R&D institutions involved, with related clinical trials reaching 131, and as many as 6734 patents.
TDM-105795 targets the TTR protein and is intended for the treatment of skin and musculoskeletal diseases, specifically alopecia. While it has reached Phase 2 globally, indicating promising results in clinical trials, it is still in Phase 1 in China, suggesting ongoing development and evaluation. Further research and trials are necessary to determine the drug's potential as a treatment option for alopecia and other related conditions.