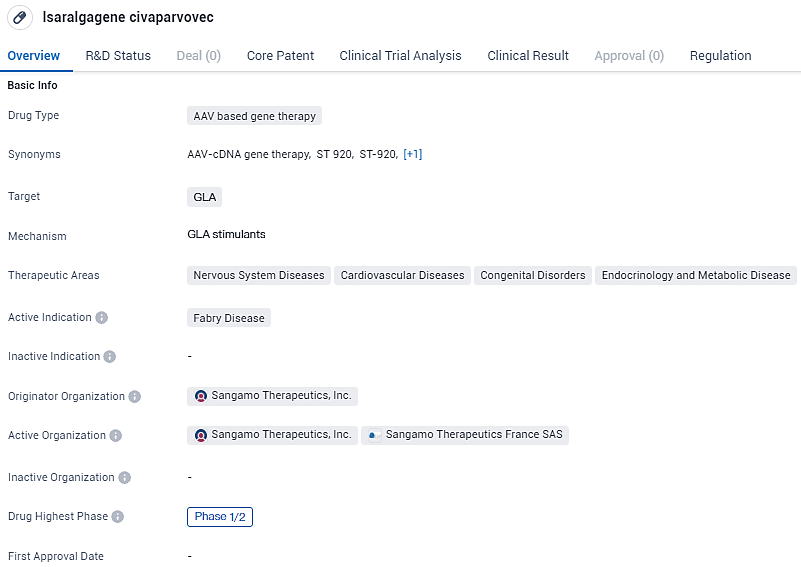
Sangamo Therapeutics Unveils Promising Phase 1/2 Results for Fabry Disease Research
Sangamo Therapeutics, Inc., a firm specializing in genomic health solutions, has disclosed recent initial results from the STAAR Phase 1/2 clinical trial. This investigation is centered on the assessment of isaralgagene civaparvovec, also referred to as ST-920, which represents an entirely proprietary gene therapeutic potential solution designed specifically to address Fabry disease.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Within the most significant clinical trial for gene therapy associated with Fabry disease known so far, information on 24 participants indicates lasting safety and provisional positive outcomes, as of the recent reporting period. These findings reinforce the prospects for isaralgagene civaparvovec as a one-time therapeutic approach for this disease.
"Even with ERT and chaperone therapy options, managing Fabry disease remains a challenge. Many patients experience progressive symptoms despite treatment. So far, the administration of ST-920 has shown good patient tolerance and early data indicates persisting, higher-than-normal levels of α-Gal A enzyme activity. The fact that patients could stop using ERT and did not have to resume is stirring enthusiasm," commented Dr. Robert Hopkin, M.D., of the Cincinnati Children’s Hospital Medical Center, and a lead investigator in the STAAR study Phase 1/2.
"Initial observations of diminished disease severity, enhanced life quality, and alleviated gastrointestinal issues, along with indications of decreased immune response reactions, suggest the promise of ST-920 as a therapy for adult Fabry disease patients," Dr. Hopkin elaborated.
Moreover, further pharmacologic and safety data concerning isaralgagene civaparvovec from the Company’s preclinical research will be displayed in other spoken presentations and a poster session during the WorldSymposiumTM. The preclinical data show heightened plasma and liver α-Gal A enzyme levels in laboratory mice models, which bolsters the dosing strategies for Phase 1/2 and potentially for a Phase 3 clinical trial phase.
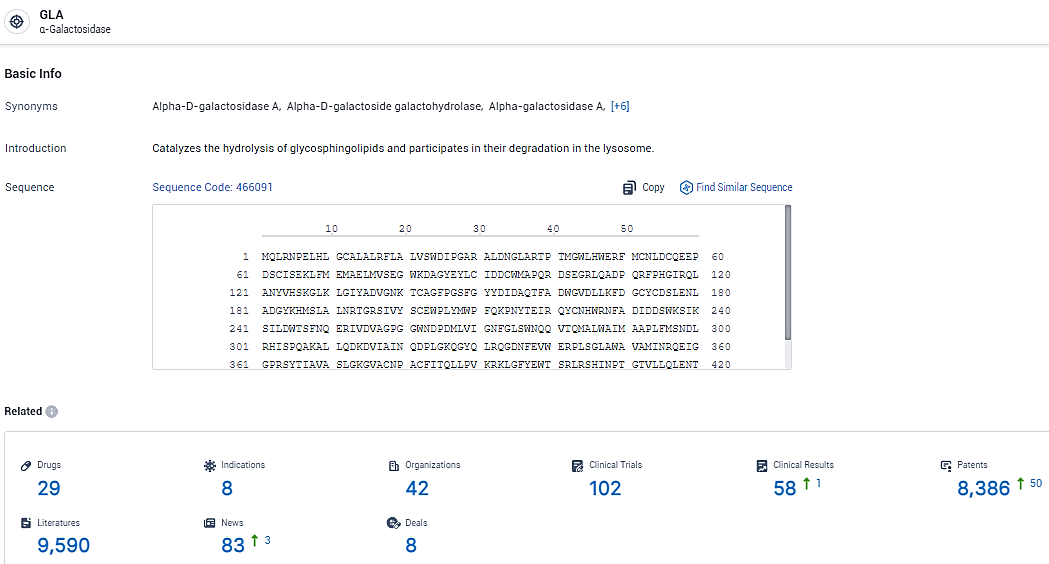
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 18, 2024, there are 29 investigational drugs for the GLA target, including 8 indications, 42 R&D institutions involved, with related clinical trials reaching 102, and as many as 8386 patents.
Isaralgagene civaparvovec shows promise as a potential treatment for Fabry Disease. However, further research and clinical trials are needed to establish its safety and efficacy. If successful, this gene therapy could provide a much-needed therapeutic option for patients with Fabry Disease, addressing the underlying cause of the condition and potentially improving their quality of life.