Exploring NDI-101150: A Promising HPK1-Targeted Therapy for Solid Tumors in Early Clinical Development
NDI-101150 is a small molecule drug developed by Nimbus Therapeutics LLC, aimed at targeting the HPK1 protein. The therapeutic focus of this drug is on neoplasms, with a specific active indication for solid tumors. As of the latest data available, NDI-101150 has reached the Phase 1/2 stage of clinical trials, indicating that it has undergone initial testing for safety and efficacy in a small group of patients.
The small molecule nature of NDI-101150 suggests that it is designed to be easily absorbed and distributed within the body, potentially allowing for targeted therapy against tumors expressing the HPK1 target. The choice of HPK1 as a target indicates a specific molecular pathway that the drug is designed to inhibit or modulate, potentially influencing the growth or spread of solid tumors.
Below, we will use the drug NDI-101150 as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
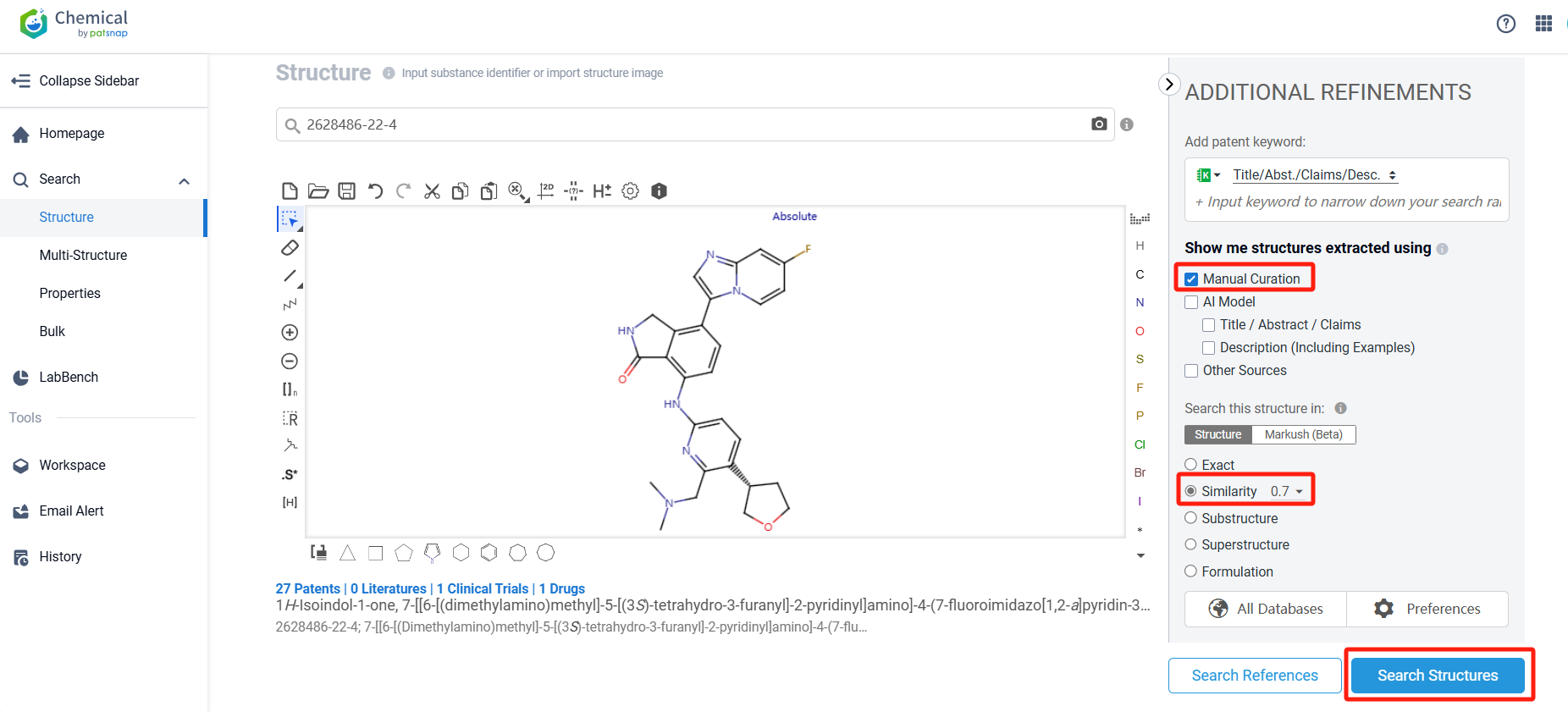
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of NDI-101150(such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, using a similarity search (setting the Tanimoto coefficient to 0.7), check the box for manual curation, click on search structures, and you can find the innovative drug NDI-101150, as disclosed in the patent application with the publication number WO2021050964A1, first made public on 2021-03-18.
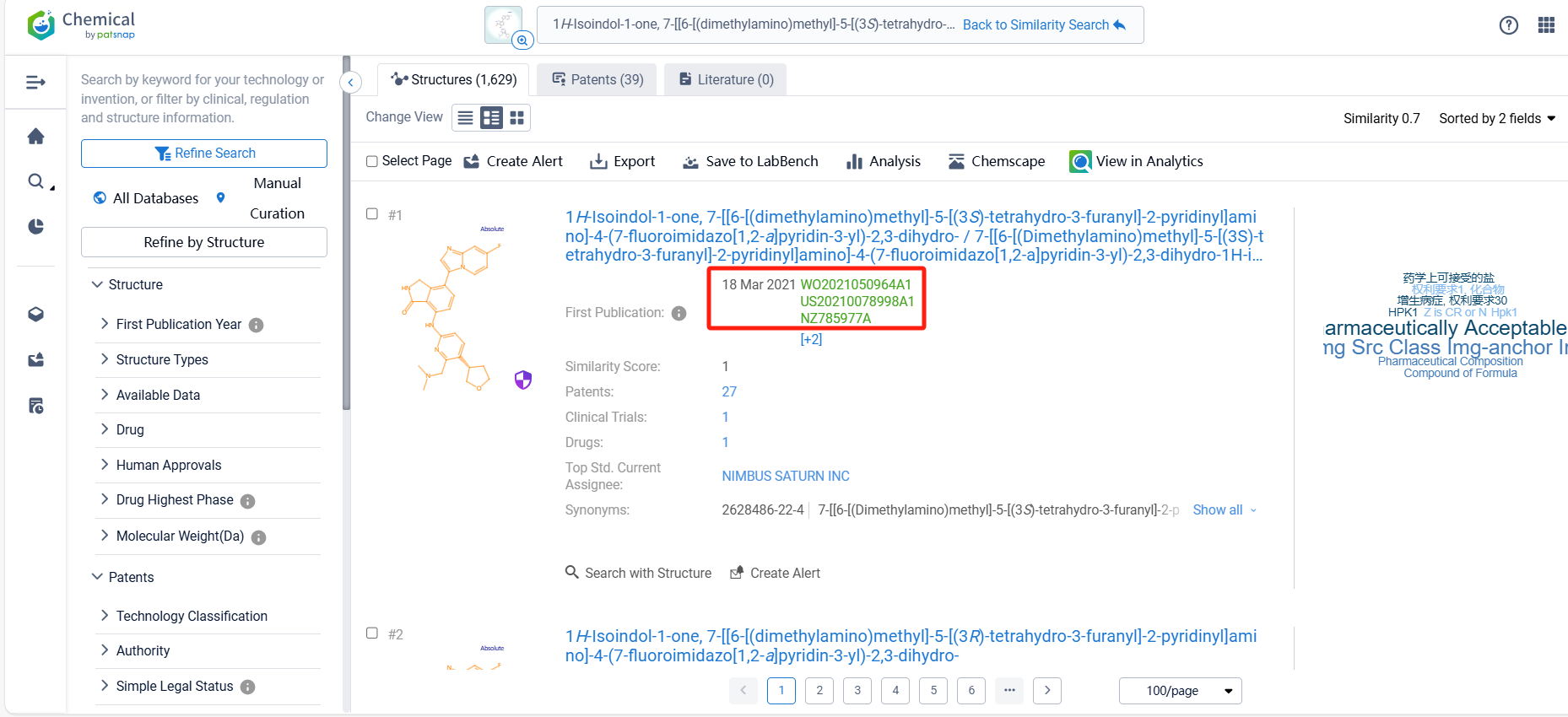
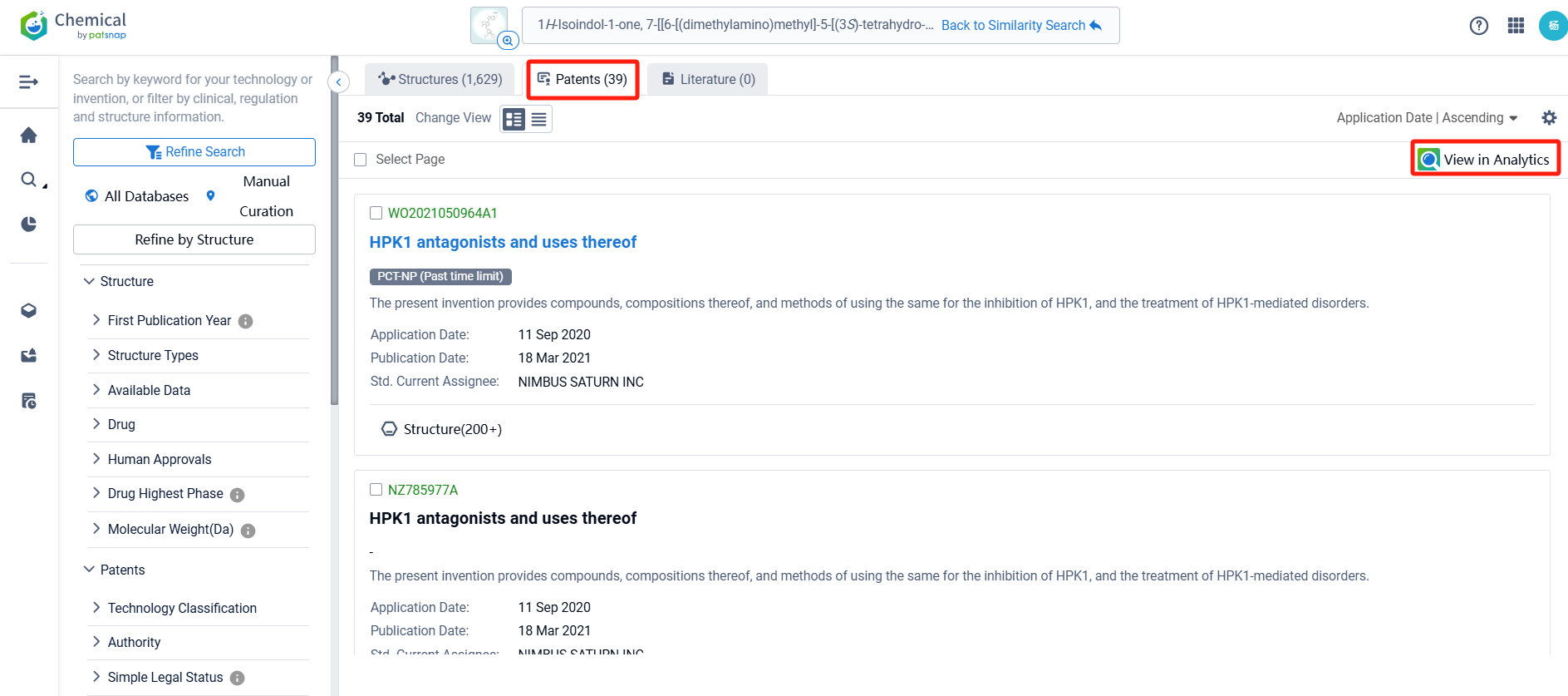
There are 39 patents related to this compound. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
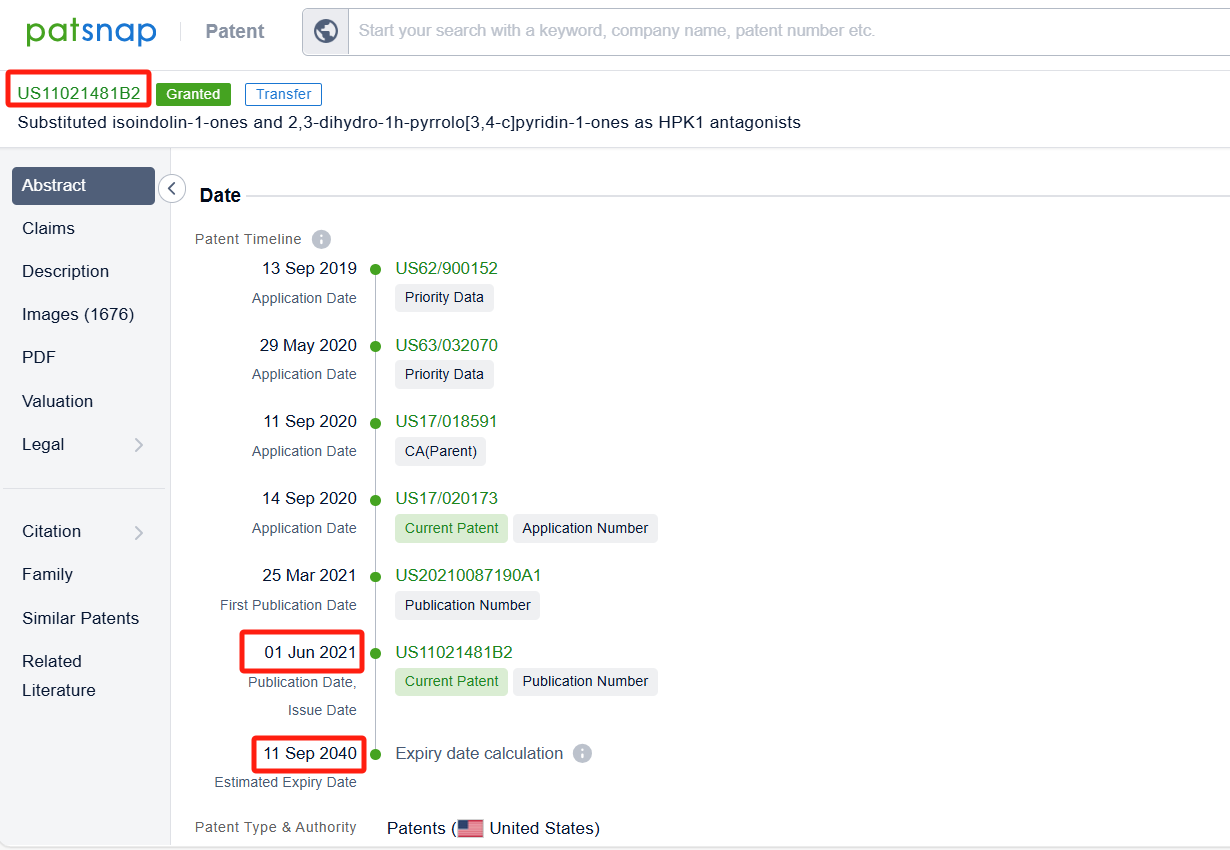
By reviewing the aforementioned patents, we can observe that the core United States patent related to this compound has been granted, with the grant publication number US11021481B2, the grant date being 01 Jun 2021, and the estimated expiration date 11 Sep 2040.
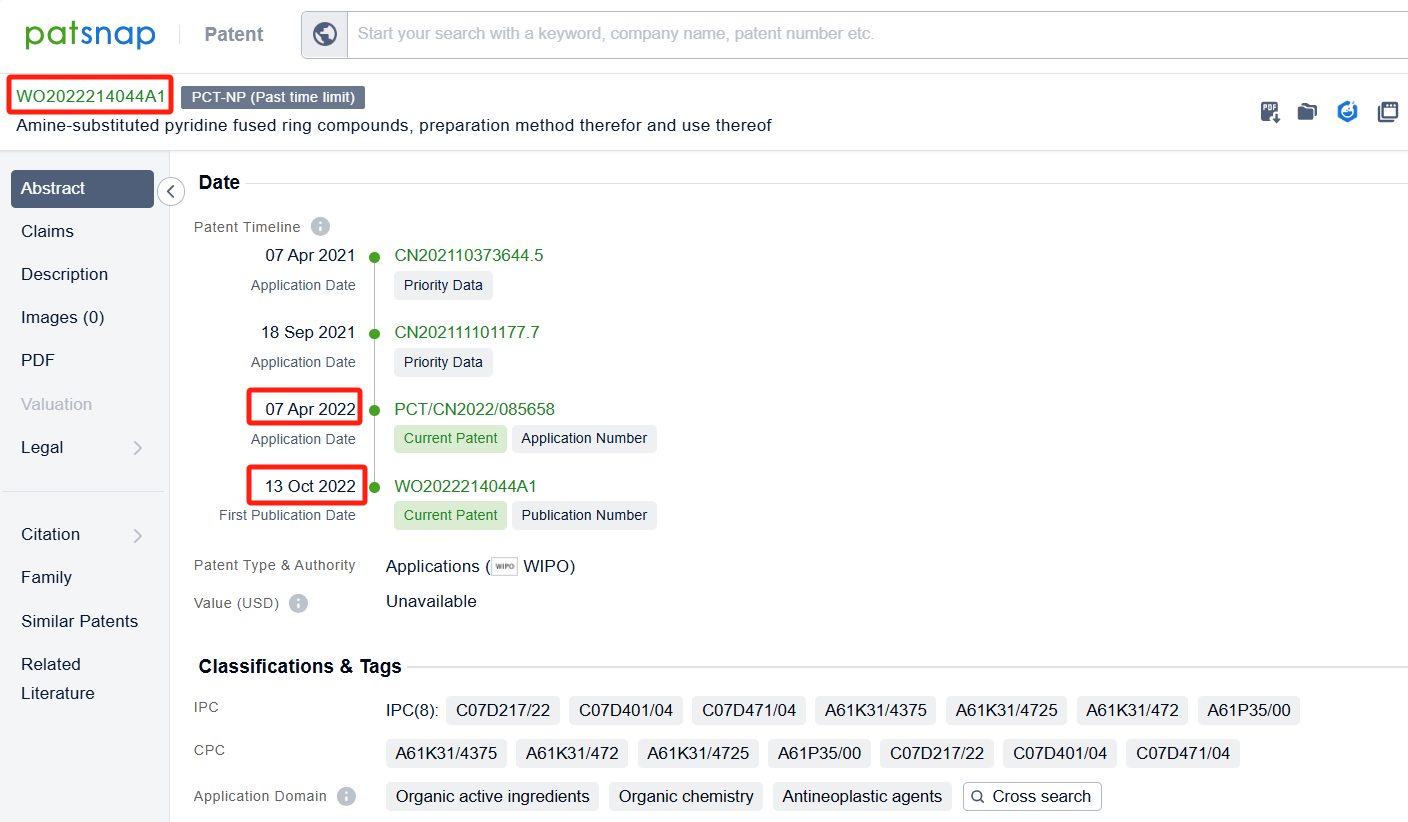
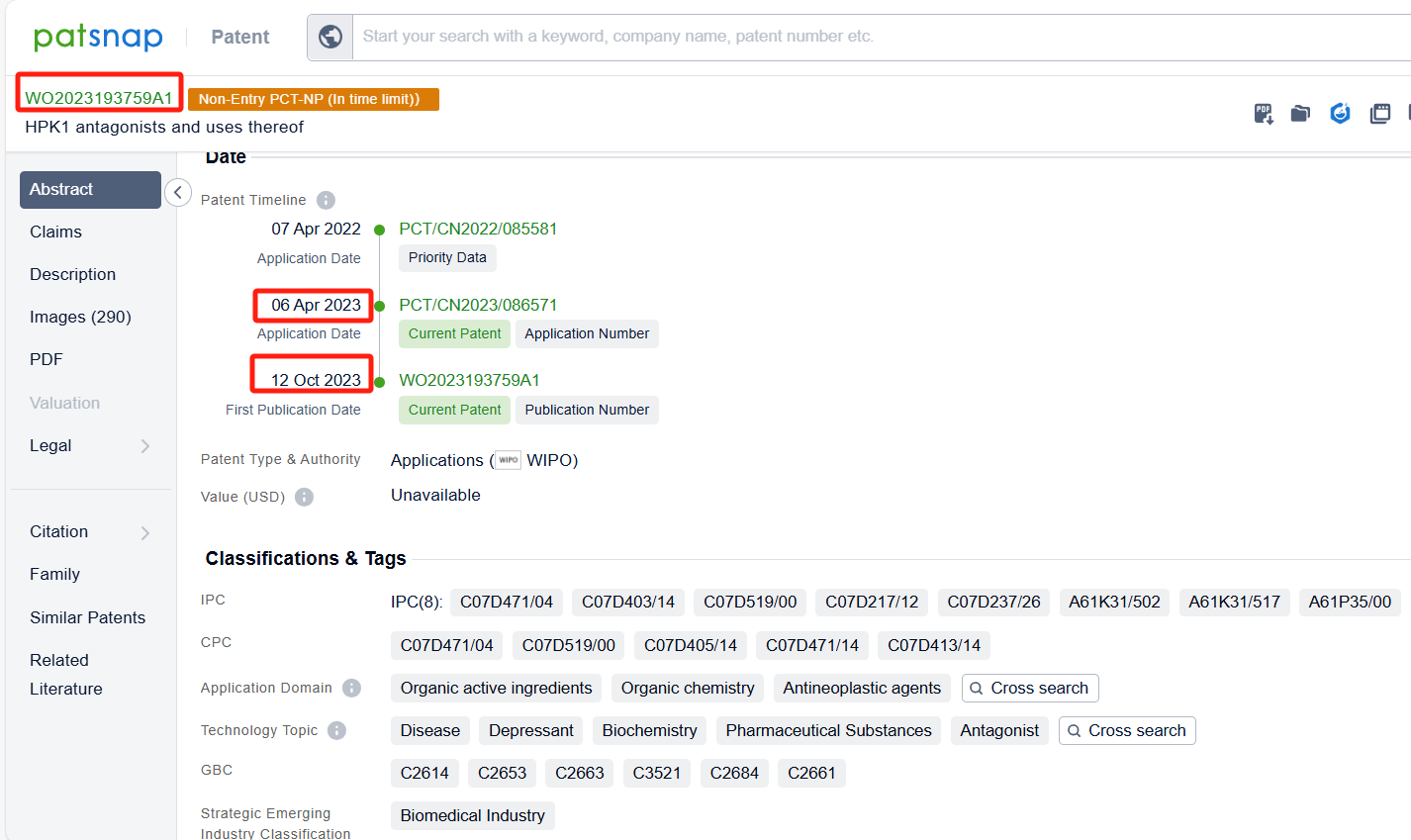
Among the applicants of the patent, one can find other companies' fast follow patents on Nimbus Saturn Inc; for example, Genfleet Therapeutics (Shanghai), Inc.'s international patent WO2022214044A1 (application date 20220407, publication date 20221013) provides a new type of highly efficient HPK1 inhibitor, which has the advantages of high activity, good selectivity, and low toxic and side effects. . Additionally, Insilico Medicine IP Ltd.'s patent WO2023193759A1 (application date 20230406, publication date 20231012) is to provide a new compound that can be used for the treatment of various medical conditions.
As the drug is still in the early phases of clinical development, further research and testing will be necessary to determine its ultimate safety and efficacy profile. If successful, NDI-101150 has the potential to offer a new treatment option for patients with solid tumors within the neoplasm therapeutic area.
In the field of Business Development, these details about NDI-101150 would be essential for evaluating potential partnerships or investment opportunities. Assessing the progress of the drug through clinical development, understanding the competitive landscape, and analyzing the potential market demand for a novel treatment targeting HPK1 in solid tumors would be key aspects of a comprehensive Business Development report in the pharmaceutical industry.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.