OncoResponse Announces Phase 1 OR502 Anti-LILRB2 Antibody Findings in Advanced Cancer Patients
OncoResponse, a biotech firm currently in the clinical trial phase, is focusing on the development of immunotherapies based on the immune systems of Elite Cancer Responders. The company has made an announcement regarding the results from the Phase 1 trial of OR502, an innovative, humanized anti-leukocyte immunoglobulin like receptor B2 (LILRB2) antibody designed to reverse immune suppression caused by LILRB2, thereby enhancing both innate and adaptive immune responses. This Phase 1 study aimed to assess the safety, tolerability, and initial anti-tumor efficacy of OR502, both as a standalone treatment and in conjunction with anti-PD-1, in patients suffering from advanced solid tumors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are delighted to report that OR502 has shown an outstanding safety profile along with encouraging early efficacy indicators. OR502 was well accepted even at the highest dosage levels, with no instances of dose-limiting toxicities (DLTs), serious adverse events (SAEs), or Grade ≥ 3 treatment-related adverse events (AEs). The study revealed promising early efficacy signals in monotherapy, including 2 partial responses (PR) and 9 stable diseases (SD) out of 17 patients, leading to a disease control rate of 65%. We look forward to advancing to the next phase of our clinical trials, which will assess OR502 in two smaller cohorts of patients suffering from cutaneous melanoma and non-small cell lung cancer (NSCLC). Completing this Phase 1 trial signifies our commitment to developing effective options to enhance the outcomes for cancer patients,” stated Clifford Stocks, CEO of OncoResponse.
“OR502 interacts with LILRB2 in a distinctive manner that counters immunosuppression in cancer, presenting a possible treatment avenue for patients who have had limited success with checkpoint inhibitor (CPI) therapies,” mentioned Kamal Puri, PhD, Chief Scientific Officer at OncoResponse. “Our team has dedicated years to creating strategies that inhibit or reprogram tumor-associated macrophages to promote anti-tumor responses. We are optimistic about the considerable potential of OR502, as initial clinical and preclinical data indicate it outperforms other anti-LILRB2 antibodies.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
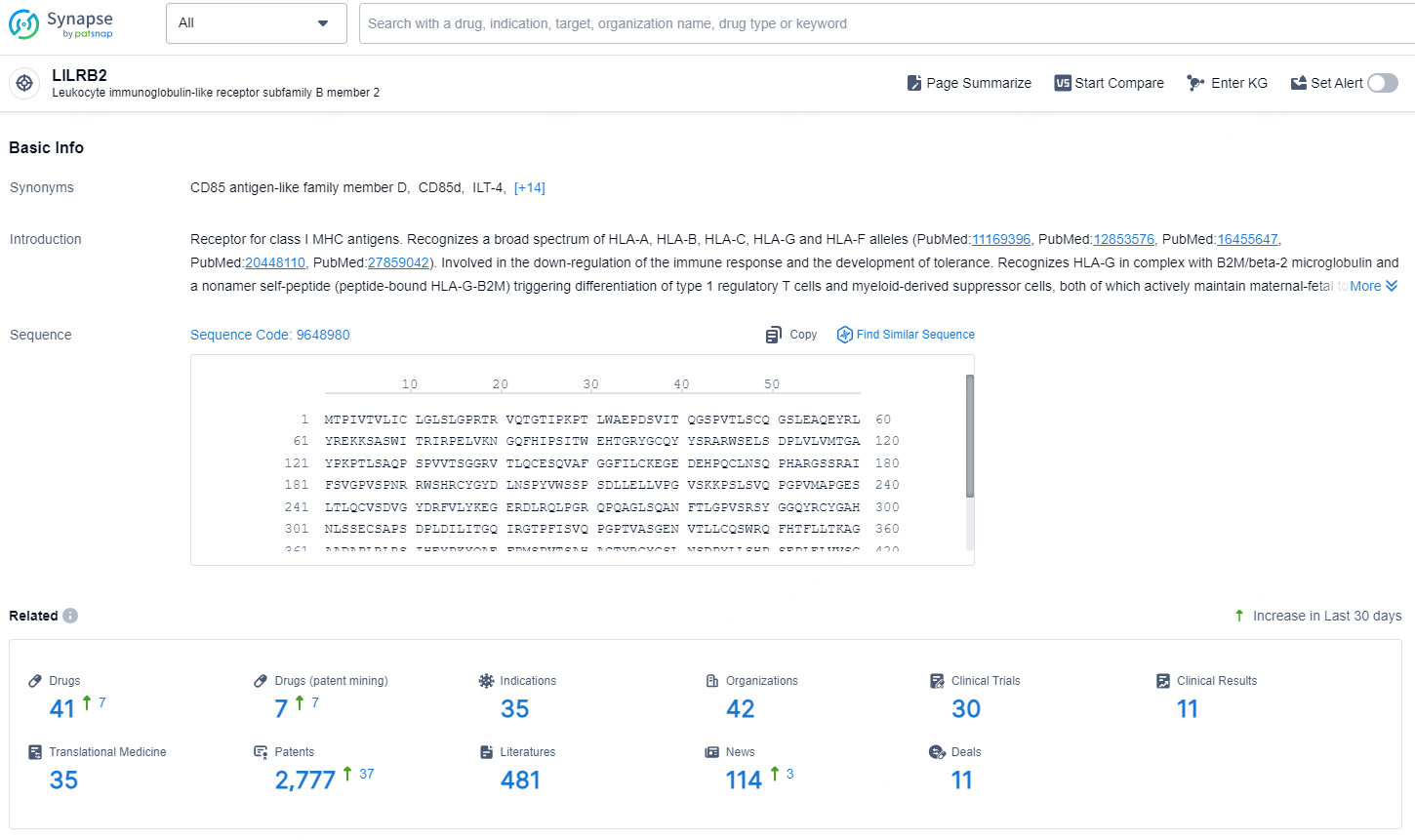
According to the data provided by the Synapse Chemical, As of November 11, 2024, there are 41 investigational drugs for the LILRB2 target, including 35 indications, 42 R&D institutions involved, with related clinical trials reaching 30, and as many as 2777 patents.
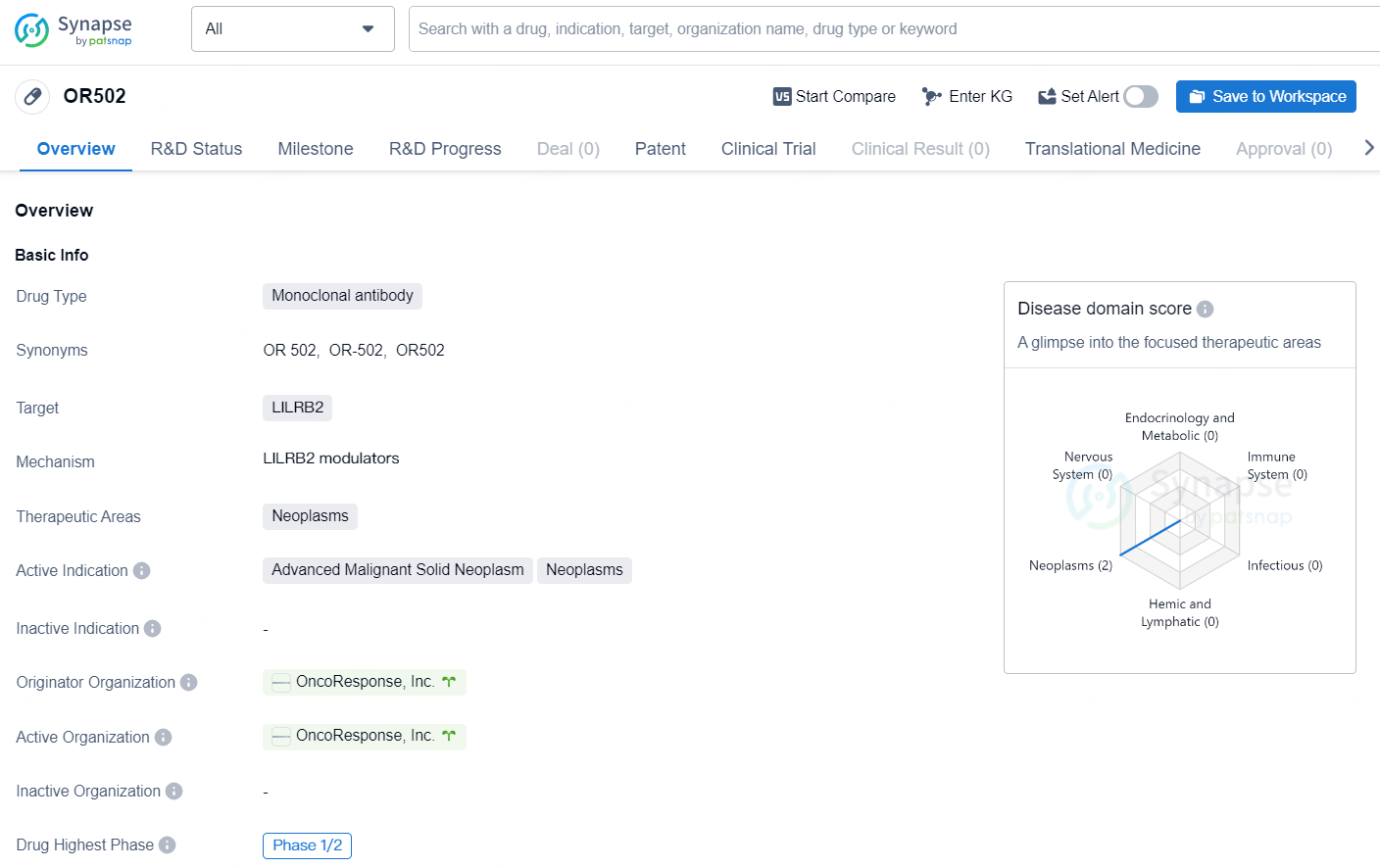
OR502 is a monoclonal antibody drug designed to target LILRB2, with a focus on therapeutic areas related to neoplasms. Its active indications are in the treatment of advanced malignant solid neoplasms and neoplasms. The drug originates from OncoResponse, Inc., and its current highest phase of development globally is at Phase 1/2.