Myeloid Therapeutics Presents MT-303 mRNA CAR for Advanced HCC at SITC 2024
Myeloid Therapeutics, Inc. ("Myeloid"), which is a clinical-stage company focused on immunology and developing RNA-based treatments for cancer, revealed that they will give an oral presentation at the 2024 annual meeting of the Society for Immunotherapy of Cancer (SITC), scheduled for November 6-10, 2024, in Houston, Texas. The presentation by Dr. Matthew Maurer, Myeloid's Chief Medical Officer, will cover the discovery and development process of MT-303. This program is Myeloid's second mRNA CAR therapy to reach clinical evaluation, originating from its portfolio of in vivo immune cell programming approaches.
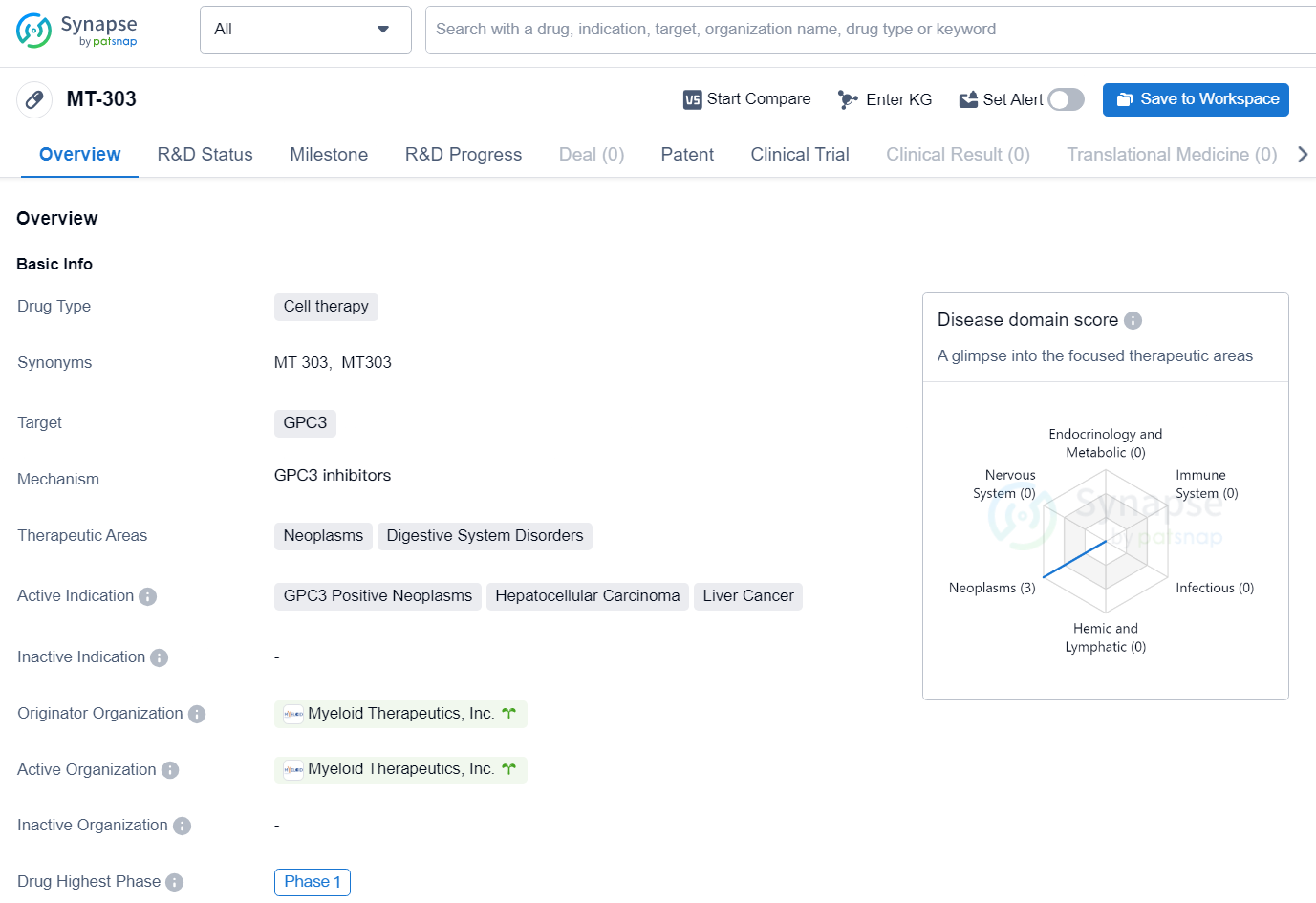
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
MT-303 is a pioneering therapy that targets Glypican-3 (GPC3) using CAR technology encoded by mRNA and delivered via lipid nanoparticles. Currently, it is being tested in a Phase 1 clinical trial for patients with advanced hepatocellular carcinoma (HCC). Treatment options for HCC are currently limited.
GPC3 is gaining attention in therapeutic research due to its high expression levels in various cancers, particularly hepatocellular carcinomas, while being minimally present in normal tissues. The presence of GPC3 is associated with more aggressive tumor characteristics and unfavorable clinical outcomes.
Myeloid’s therapeutic strategy involves the targeted in vivo programming of specific immune cell subsets using innovative mRNA-encoded CAR solutions that do not require preconditioning. MT-303 equips myeloid cells with a unique chimeric antigen receptor that is designed to function specifically within these cells. This strategic approach allows myeloid cells to target and eliminate GPC3-expressing tumors, while also fostering a unified adaptive immune response characterized by the proliferation of new T cell clones.
"Myeloid has quickly progressed MT-303 to first-in-human trials as our second in vivo CAR clinical initiative. We continue to escalate doses of MT-303 and MT-302 and are encouraged by the ongoing biological validation of mechanisms, along with initial safety and efficacy observations," said Dr. Matthew Maurer, the Chief Medical Officer of Myeloid. "These clinical findings build on earlier preclinical studies, which also showed selective activation of CAR myeloid cells and significant anti-tumor effects in animal models of hepatocellular tumors."
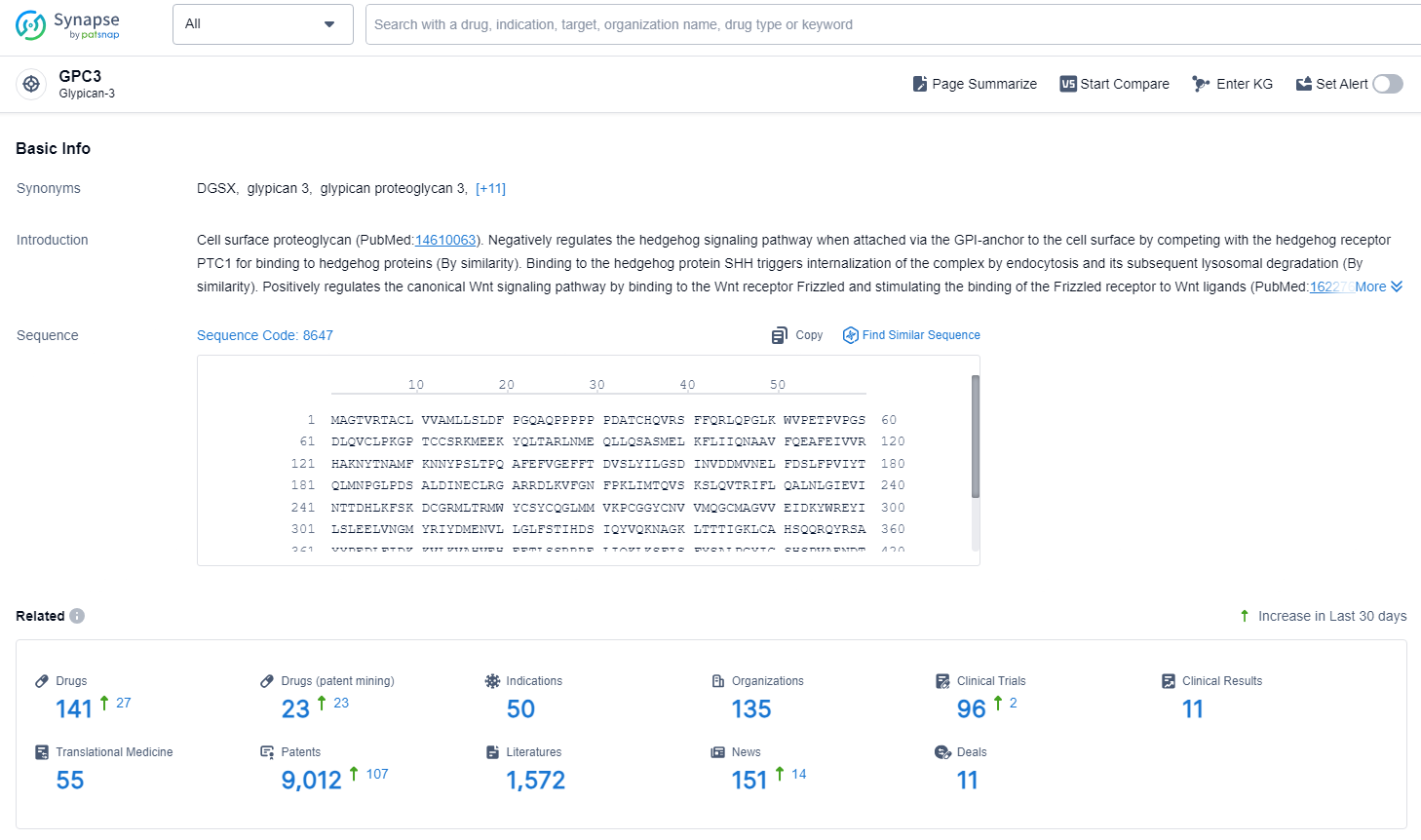
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 13, 2024, there are 141 investigational drugs for the GPC3 target, including 50 indication, 135 R&D institutions involved, with related clinical trials reaching 96, and as many as 9012 patents.
MT-303 is a cell therapy drug developed by Myeloid Therapeutics, Inc. It is targeted towards GPC3, with therapeutic areas including neoplasms and digestive system disorders. The drug is specifically indicated for GPC3 positive neoplasms, hepatocellular carcinoma, and liver cancer.