Rapid Growth and Advancements in AAV Gene Therapy: Market, Mechanism, and Clinical Applications
In 2023, the global market for AAV (Adeno-associated virus) therapies reached $1.5 billion, with projections suggesting growth to $22.3 billion by 2029, underscoring the rapid development of AAV therapies. Currently, eight AAV gene therapies have been approved worldwide, with over 900 pipeline projects in progress, highlighting both the swift expansion and widespread research interest in AAV gene therapies. AAV gene therapy holds a central position in the field due to its efficient, safe, and durable gene delivery capabilities. With recent advances in understanding the biological properties of AAV, gene engineering techniques, and accumulated clinical trial data, AAV gene therapies are entering a period of rapid global growth.
Mechanism of Action of AAV Vectors
As an effective gene therapy vector, AAV operates through a complex sequence of steps, from binding and entry at the cell surface to eventual gene expression and protein synthesis. AAV binds to specific receptors on the host cell surface via surface proteins, a process crucial for the virus's targeted infection. For example, AAV2 primarily recognizes and attaches to heparan sulfate proteoglycans (HSPG) on host cells, which aids in cellular attachment and entry. This binding facilitates virus adsorption and triggers intracellular signaling pathways, leading AAV to enter the cell through receptor-mediated endocytosis.
AAV binds to specific receptors on the host cell surface through its surface proteins, a critical process for the virus’s selective infection. For instance, AAV2 primarily attaches to heparan sulfate proteoglycans (HSPG) on the host cell surface, which facilitates cellular recognition and attachment. This binding not only aids in viral adhesion but also activates intracellular signaling pathways, triggering receptor-mediated endocytosis that allows AAV to enter the cell. Endocytosis typically relies on clathrin or other mechanisms like caveolin or micropinocytosis, assisting viral particles in reaching the cell interior. Once AAV particles enter through endocytosis, they are enclosed in endosomes. The acidic environment within the endosomes induces major structural changes in the viral capsid, exposing a phospholipase A2 (PLA2) catalytic domain. This exposure enables viral particles to escape into the cytoplasm. The escape process can vary based on cell type and AAV serotype, with different serotypes exhibiting varying cellular affinities, allowing enhanced transduction efficiency in specific tissues or cell types through appropriate serotype selection.
Once in the cytoplasm, AAV particles use the cell's microtubule transport system to approach the nucleus. This process occurs through interactions with microtubules and motor proteins such as dynein and kinesin. The microtubule transport system plays a crucial role in intracellular directional movement, efficiently transporting AAV particles to the vicinity of the nucleus to prepare for nuclear entry. When AAV particles near the nucleus, they interact with nuclear pore complexes (NPCs) and are transported through NPCs into the nucleus. NPCs are large molecular channels on the nuclear membrane that regulate nuclear transport. The nuclear import of AAV is a tightly regulated process, involving interactions between various cellular factors and viral proteins. NPCs consist of multiple protein subunits forming a channel approximately 100 nanometers in diameter. These subunits include nucleoporins, which are essential to NPC structure and function. FG repeat sequences in nucleoporins (phenylalanine-glycine repeats) form a selective filter, allowing only specific proteins and macromolecular complexes to pass through and enter the nucleus.
Some research indicates that AAV particles may enter the nucleus intact through NPCs. This process depends on interactions between viral capsid proteins, particularly VP1 and VP2, and FG repeat sequences in nucleoporins. After entering the nucleus, the viral particles undergo uncoating to release their single-stranded DNA genome. Another view suggests that AAV particles may partially uncoat in the cytoplasm, releasing portions of viral DNA. These exposed DNA fragments then enter the nucleus through NPCs and undergo further uncoating and processing. Regardless of the mechanism, once inside the nucleus, AAV’s DNA must be unpackaged for subsequent processing. This involves the breakdown and removal of viral capsid proteins, potentially facilitated by host cell proteases and other enzymes, exposing the single-stranded DNA genome. Upon entry, the single-stranded DNA genome undergoes secondary structural changes to form double-stranded DNA, either through host DNA repair mechanisms or replication-aiding factors within the viral genome. In double-stranded form, the AAV genome utilizes inverted terminal repeats (ITRs) for inter- or intramolecular genomic recombination. This recombination stabilizes the AAV genome as episomal DNA (non-integrating circular DNA), enabling sustained gene expression in non-dividing cells. The ITR sequences within the AAV genome drive this recombination process, forming a circular, episomal genome that can persist in the nucleus, promoting continuous transgene expression and protein synthesis.
Market Drivers for AAV Technology
Many genetic disorders currently lack effective treatments, particularly those caused by single-gene mutations, such as cystic fibrosis, Huntington's disease, spinal muscular atrophy (SMA), and Duchenne muscular dystrophy (DMD). AAV (Adeno-Associated Virus) technology brings new hope for treating these conditions by restoring or improving patients’ physiological functions through gene replacement or gene editing. Although rare disease patient populations are relatively small individually, they are substantial in total. Statistics show there are over 7,000 rare diseases worldwide, affecting millions of people. The application of AAV technology in rare disease treatment offers more therapeutic options for these patients, driving market demand growth. Large-scale production of AAV vectors has long been a challenge in gene therapy. Recently, improvements in production processes, such as the use of suspension cultures and bioreactors, and new manufacturing technologies (like TESSA™ technology), have significantly enhanced AAV vector yield and purity, reducing production costs and advancing AAV commercialization.
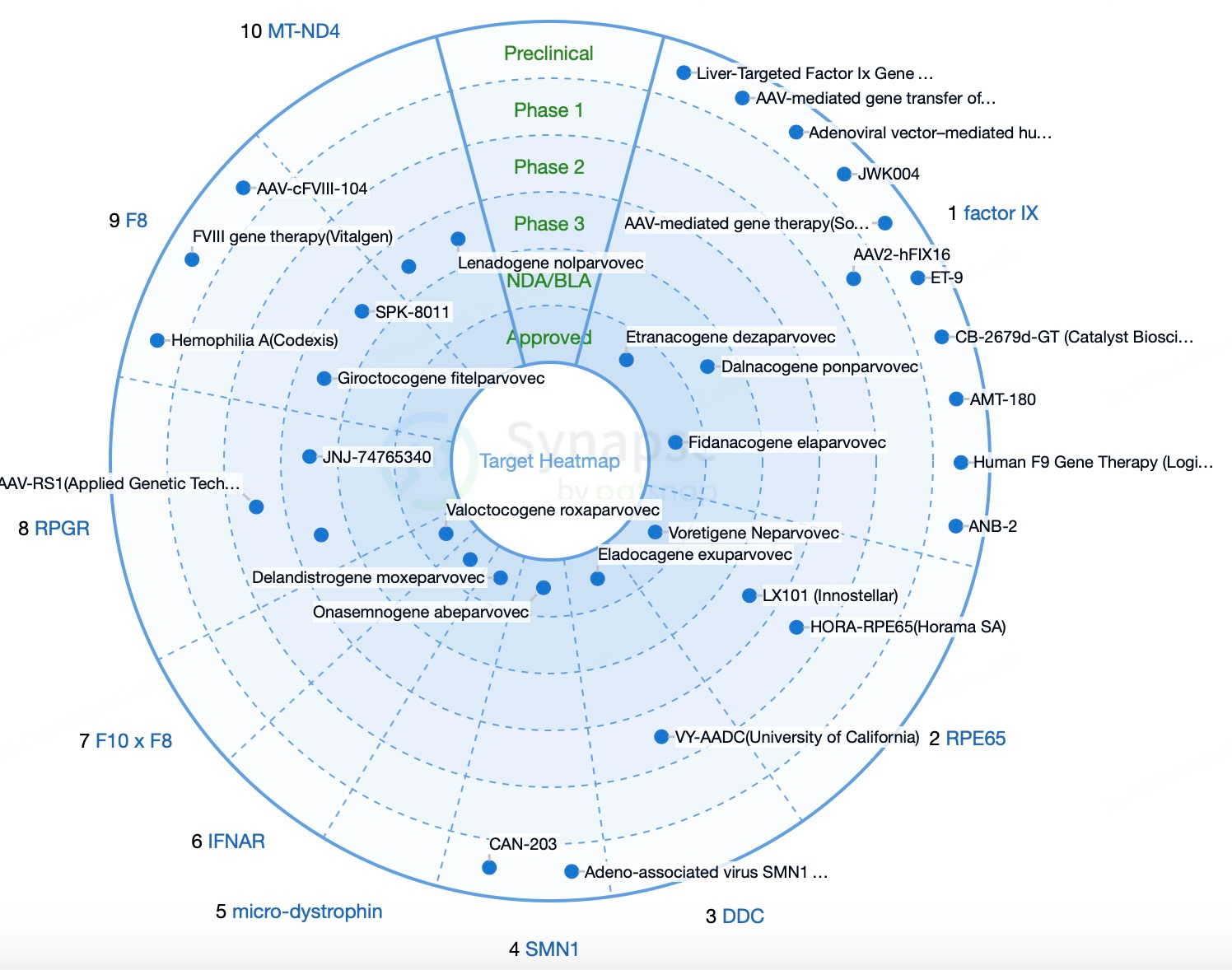
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) provide accelerated approval pathways for gene therapy products. These policies aim to speed up the market availability of innovative therapies to meet urgent patient needs, thus promoting the development of AAV technology. Companies in gene therapy are accelerating research and commercialization through partnerships and alliances to share resources and technologies. For example, Sarepta Therapeutics collaborates with various research institutions and pharmaceutical companies to advance the application of AAV technology in DMD treatment. Traditional gene therapy products are often personalized and customized, resulting in high production costs and long timelines. Recent innovations, such as using universal CAR-T cells and AAV vectors, have enabled the development of “off-the-shelf” therapies. These can be produced in bulk in advance and applied to broader patient groups, lowering costs and improving accessibility.
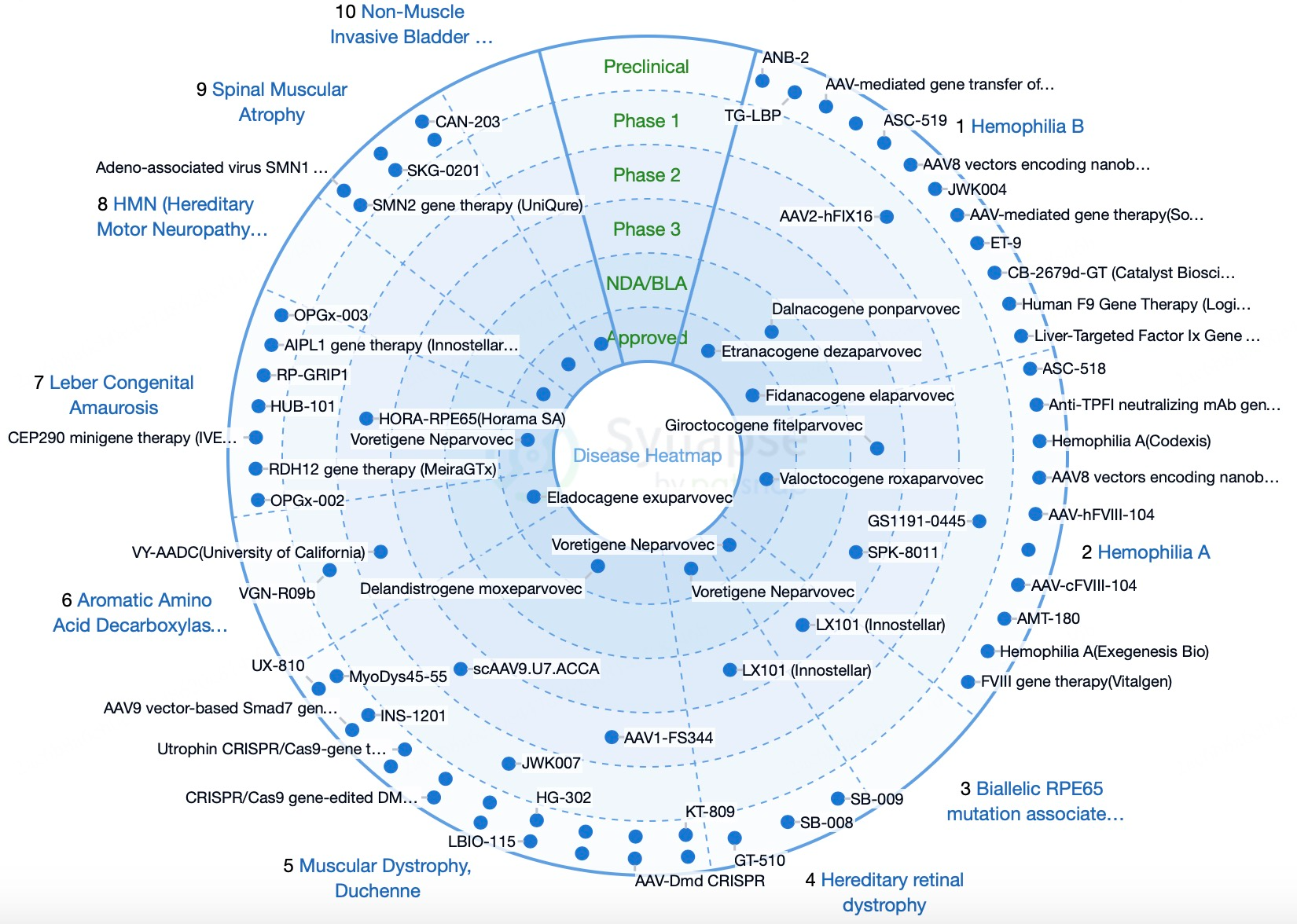
Regional Analysis of AAV Technology Development
North America and Europe lead globally in AAV technology research and applications, supported by strong research institutions, biotechnology companies, and comprehensive policy frameworks with significant market potential. The U.S. and the EU facilitate rapid AAV product approvals through FDA and EMA’s accelerated pathways, playing a key role in innovation and commercialization in gene therapy.
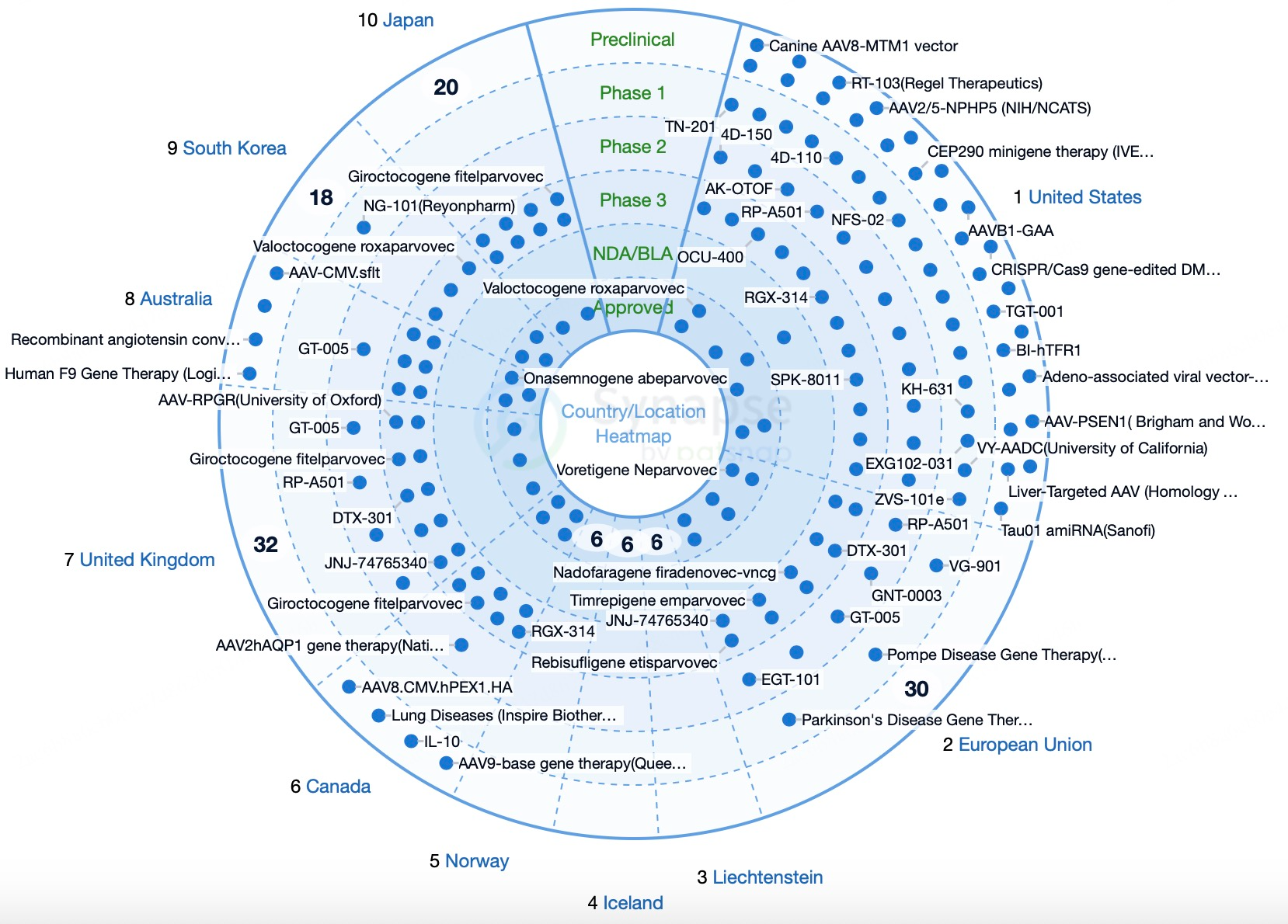
In Asia, particularly China and Japan, there is rapid growth as an important hub for AAV development. The Chinese government supports gene therapy research and industrialization through various policies, while Japan promotes clinical applications of AAV products with its advanced technology and favorable market environment. Other regions, such as Australia, the Middle East, and Africa, though still in early stages, show promising development potential. International collaborations and policy support are gradually advancing AAV technology development in these areas.
Market Competitive Landscape for AAV Technology
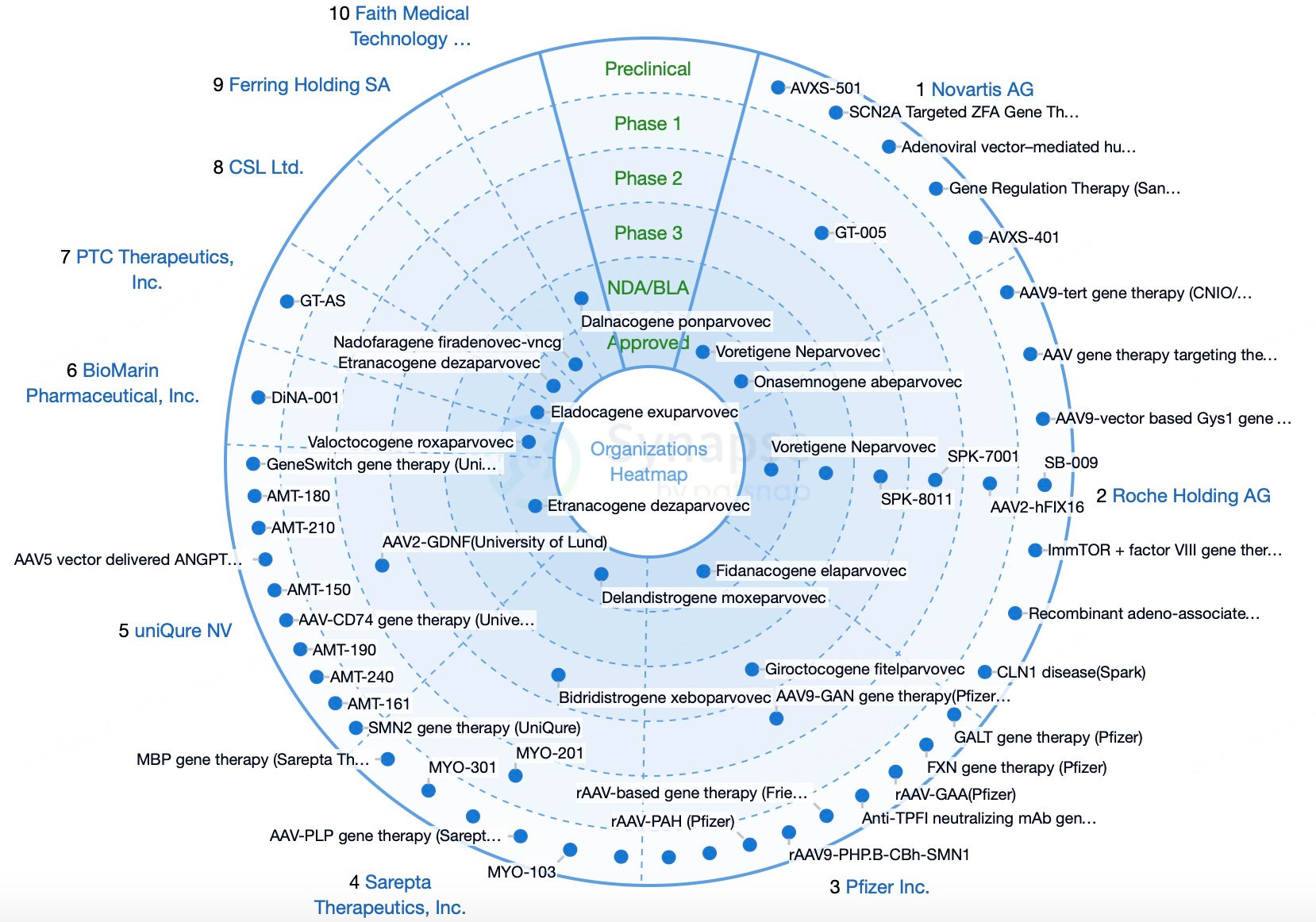
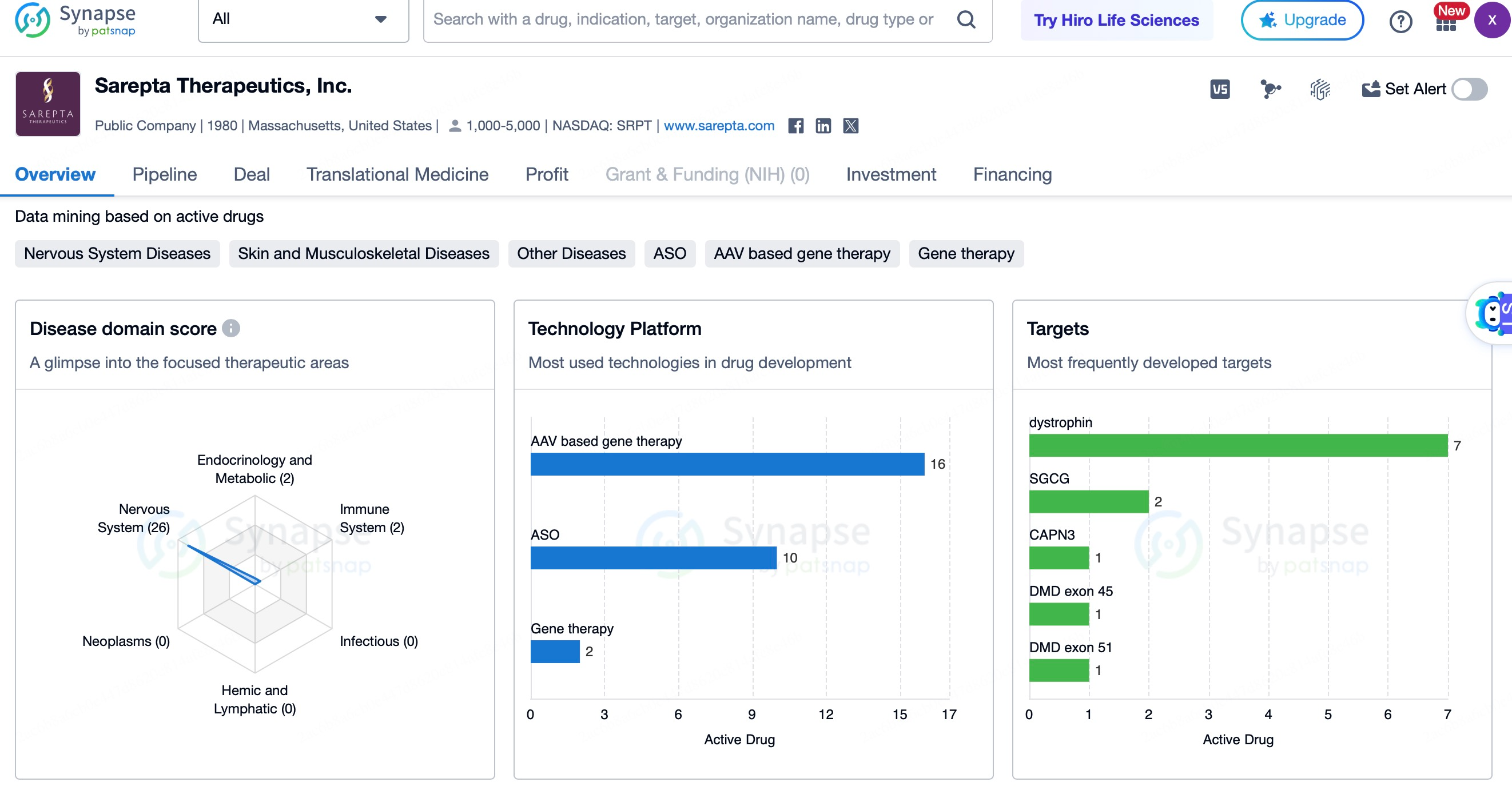
Sarepta Therapeutics
Sarepta Therapeutics is a leading biopharmaceutical company dedicated to developing and commercializing innovative therapies for rare diseases, particularly in the gene therapy field. The company has made significant progress with AAV gene therapies, notably in Duchenne muscular dystrophy (DMD) and limb-girdle muscular dystrophy (LGMD). Its AAV-based gene therapy product SRP-9001 (Elevidys) has undergone several clinical trials and received FDA accelerated approval in 2023 for treating DMD in children aged 4-5 with dystrophin gene mutations. Despite Phase III trials not achieving their primary endpoint, the therapy demonstrated clinically meaningful benefits, generating $200.4 million in sales in 2023. Sarepta is also developing other DMD therapies, such as Amondys 45 and Vyondys 53, targeting exon-skipping therapies for exons 45 and 53. In LGMD, Sarepta’s SRP-9003 has entered clinical trials, showing promising safety and efficacy.
Spark Therapeutics
Spark Therapeutics is a biotechnology leader in gene therapy, particularly for ophthalmic diseases. Its landmark product Luxturna (voretigene neparvovec-rzyl) is FDA-approved to treat Leber congenital amaurosis (LCA) due to RPE65 mutations. Luxturna, the first approved "in vivo" gene therapy, delivers a functional RPE65 gene to retinal cells through a single intravitreal injection, restoring visual function. This approval marked a significant milestone in gene therapy, offering new hope for patients with inherited vision loss. Spark is also developing gene therapies for other genetic diseases, including hemophilia A, lysosomal storage disorders, and neurodegenerative diseases, demonstrating its extensive capabilities and innovation in the gene therapy landscape.
uniQure
Based in the Netherlands, uniQure is a biotechnology company focused on developing AAV-based gene therapies, achieving notable success in rare and neurological disorders. Key products include Glybera, the world's first approved gene therapy, for lipoprotein lipase deficiency (LPLD). Despite being withdrawn in 2017 due to high costs and limited market acceptance, it showcased the potential of AAV-based therapies. uniQure’s AMT-130 targets Huntington's disease (HD), using AAV5 to deliver a microRNA that silences the Huntington (HTT) gene, thus slowing disease progression. In August 2023, Phase I/II clinical trial results highlighted its potential in reducing disease progression. Additionally, Hemgenix (etranacogene dezaparvovec), an AAV5-based gene therapy for hemophilia B, received FDA approval in November 2022, marking it as the first approved AAV gene therapy for hemophilia B.
Oxford BioMedica
Headquartered in the UK, Oxford BioMedica is a gene therapy company specializing in lentiviral and AAV vector development, with products targeting genetic diseases and cancer. Established in 1995, Oxford BioMedica has become a global player in cell and gene therapy, largely due to its Lentiviral Vector (LVV) platform. This technology efficiently delivers therapeutic genes into target cells and is applicable for treating neurodegenerative diseases, ophthalmic disorders, and cancers. Oxford BioMedica collaborated with Novartis to develop Kymriah (tisagenlecleucel), the first approved CAR-T cell therapy for B-cell acute lymphoblastic leukemia. The company is also developing gene therapies, such as AXO-Lenti-PD for Parkinson’s disease and BI 3720931 for cystic fibrosis, through partnerships with other organizations.
Summary
AAV, as a non-replicating, non-enveloped, single-stranded DNA virus, offers advantages like high safety, long-term expression, and broad application potential in gene therapy. Its stability, minimal host cell impact, and ability to deliver therapeutic genes with sustained expression make AAV vectors promising for durable therapeutic effects. Once in the cell nucleus, AAV sheds its capsid and releases its single-stranded genome, which converts into a double-stranded DNA template, allowing transgene transcription and translation to express the therapeutic gene. There are 13 natural serotypes of AAV (AAV1-13) and over 200 variants, each with distinct tissue tropisms, making it possible to target different tissues. Significant progress in AAV gene therapy has been made in clinical applications, treatment effectiveness, and therapy innovation. With ongoing advancements, AAV gene therapy is positioned to redefine the future of gene-based treatments, offering more therapeutic options and hope to patients worldwide.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Refrence
- 1.Dhungel BP, Winburn I, Pereira CDF, Huang K, Chhabra A, Rasko JEJ. Understanding AAV vector immunogenicity: from particle to patient. Theranostics. 2024 Jan 20;14(3):1260-1288. doi: 10.7150/thno.89380. PMID: 38323309; PMCID: PMC10845199.
- 2.Ling Q, Herstine JA, Bradbury A, Gray SJ. AAV-based in vivo gene therapy for neurological disorders. Nat Rev Drug Discov. 2023 Oct;22(10):789-806. doi: 10.1038/s41573-023-00766-7. Epub 2023 Sep 1. PMID: 37658167.
- 3.Jiang Z, Dalby PA. Challenges in scaling up AAV-based gene therapy manufacturing. Trends Biotechnol. 2023 Oct;41(10):1268-1281. doi: 10.1016/j.tibtech.2023.04.002. Epub 2023 Apr 30. PMID: 37127491.