Exploring NXP-800's R&D successes and its clinical results at the 2024 AACR
According to the previous study, NXP-800 caused growth inhibition and regression in various preclinical models, including ARID1A mutated ovarian and endometrial xenograft and cholangiocarcinoma PDX models. On April 5, 2024, the first in human clinical trial of NXP-800 was reported in 2024 AACR.
NXP-800's R&D Progress
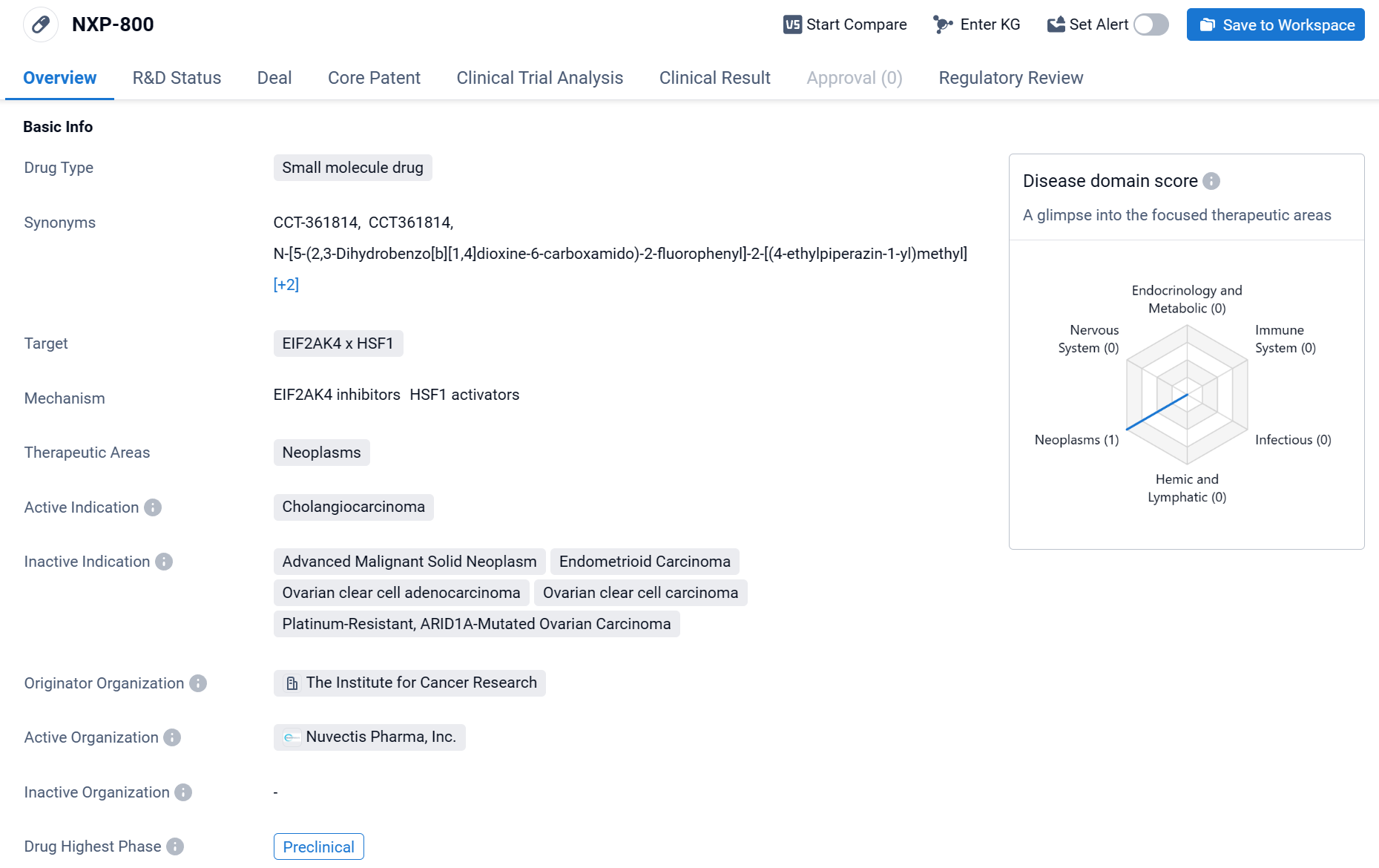
NXP-800 is a small molecule drug that is being developed in the field of biomedicine. It targets EIF2AK4 x HSF1 and is primarily focused on treating neoplasms, specifically cholangiocarcinoma.
According to the Patsnap Synapse, NXP-800 is currently in the preclinical phase. And the clinical trial distributions for NXP-800 are primarily in the United State and United Kingdom. The key indication is Advanced Malignant Solid Neoplasm.
Detailed Clinical Result of NXP-800
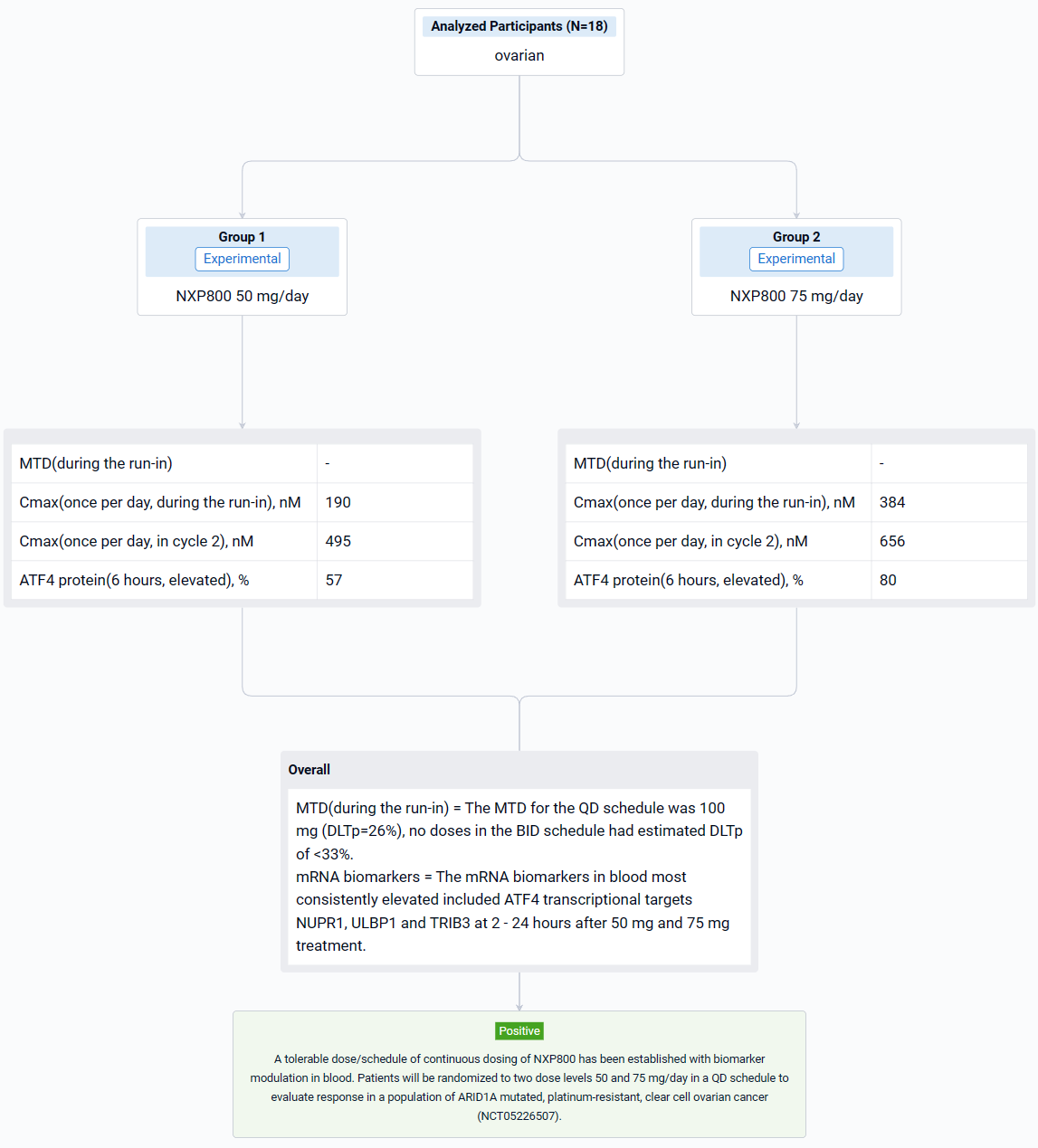
This non-randomized, sequential assignment, open-labeled clinical study (NCT05226507) described the dose-defining dose escalation cohort and the toxicity-pharmacokinetics and pharmacodynamic evaluation in a first-in-human phase I trial of NXP800.
In this study, dose escalation was guided by a Bayesian modified continual reassessment method that targeted a dose-limiting toxicity DLT probability (DLTp) closest to 30% but <33%. Toxicity was assessed using NCI.CTC.V5. Detailed sampling of blood for pharmacokinetic analysis and blood for mRNA and protein analysis in peripheral blood mononuclear cells was conducted on C1D-3 to -7 (run in prior to continuous dosing) and C2D1. Two schedules (once per day [QD] and twice per day [BID] and a dose range of 50 to 150 mg/day were evaluated.
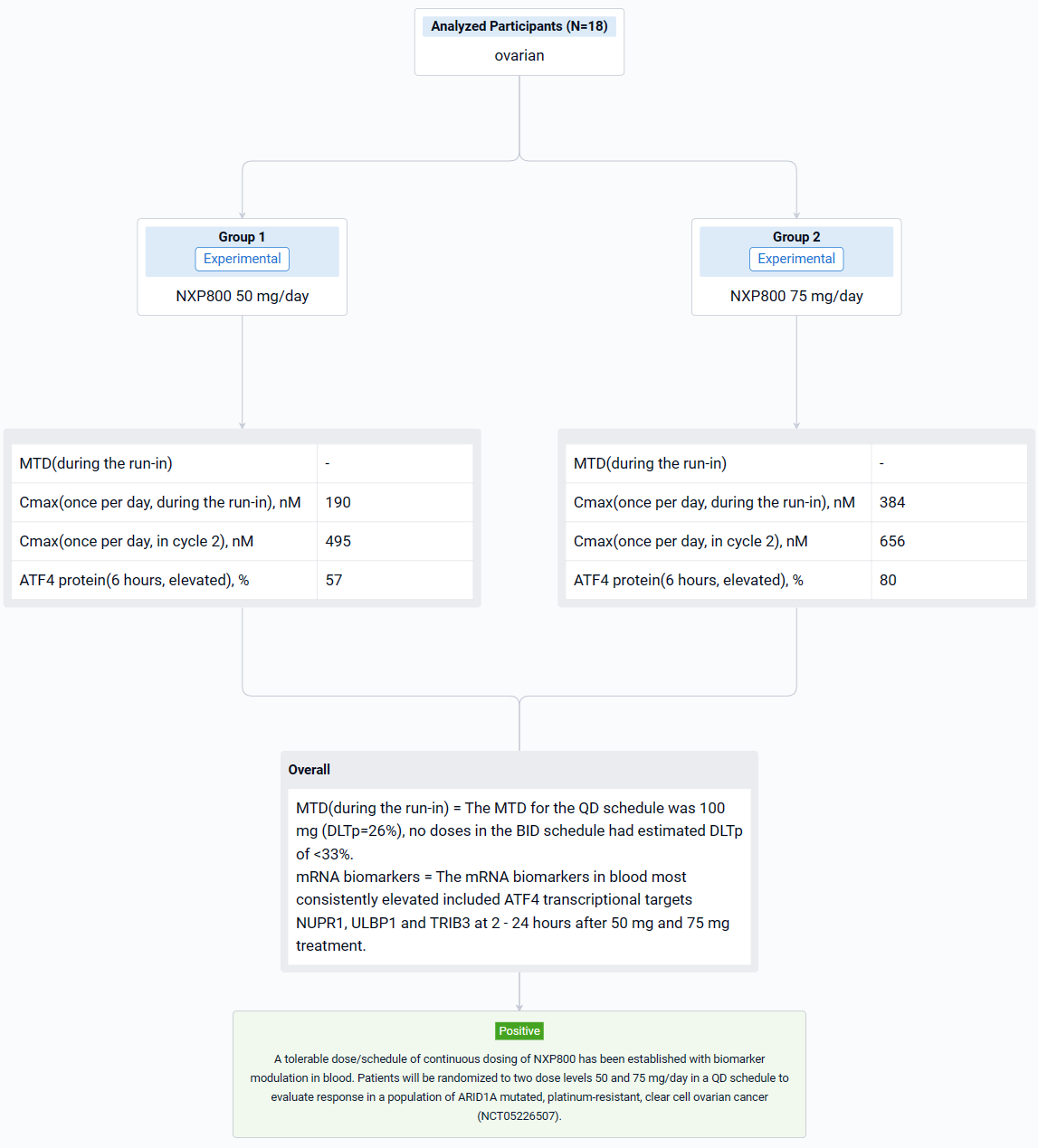
The result showed that eighteen patients were treated: at baseline, the median age was 65 years (range 42-77), 9 (50%) were female and 14/18 (78%) had an ECOG score of 1. The most common treatment emergent adverse events (more than 20% of patients) were nausea, vomiting, diarrhea, fatigue, decreased appetite, AST increase and thrombocytopenia. All these side effects were grade 1-2 except grade 3 nausea and grade 3 diarrhea reported in one patient each and grade 3 thrombocytopenia in 2 patients. The MTD for the QD schedule was 100 mg (DLTp=26%), no doses in the BID schedule had estimated DLTp of <33%. The average Cmax at 50 mg and 75 mg OD was 190 nM and 384 nM during the run-in and 495 nM and 656 nM in cycle 2 reached by 3 hours. ATF4 protein in PBMCs was elevated in 57% and 80% of patients during run in and Cycle 2 respectively, 6 hours after 50 mg and 75 mg treatment. The mRNA biomarkers in blood most consistently elevated included ATF4 transcriptional targets NUPR1, ULBP1 and TRIB3 at 2 - 24 hours after 50 mg and 75 mg treatment. Further, mRNA levels for these biomarkers were elevated in post-treatment biopsy samples in one of the two patients tested. It is envisaged that NXP800 will need to be dosed continuously and although the protocol defined MTD at 28 days at 100 mg QD, it was decided to take a dose of range of 50 mg and 75 mg OD which caused biomarker modulation, forward for further evaluation.
It can be concluded that a tolerable dose/schedule of continuous dosing of NXP800 has been established with biomarker modulation in blood. Patients will be randomized to two dose levels 50 and 75 mg/day in a QD schedule to evaluate response in a population of ARID1A mutated, platinum-resistant, clear cell ovarian cancer (NCT05226507).
How to Easily View the Clinical Results Using Synapse Database?
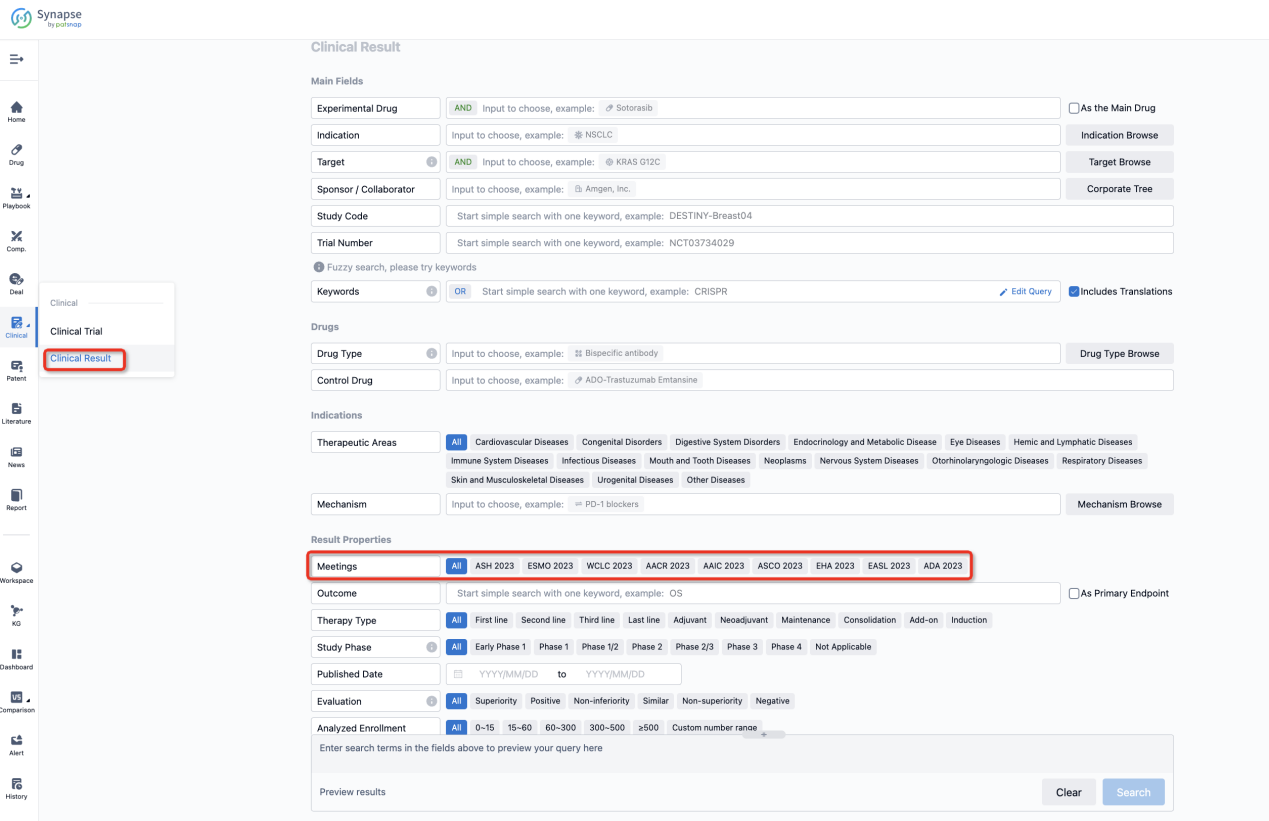
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
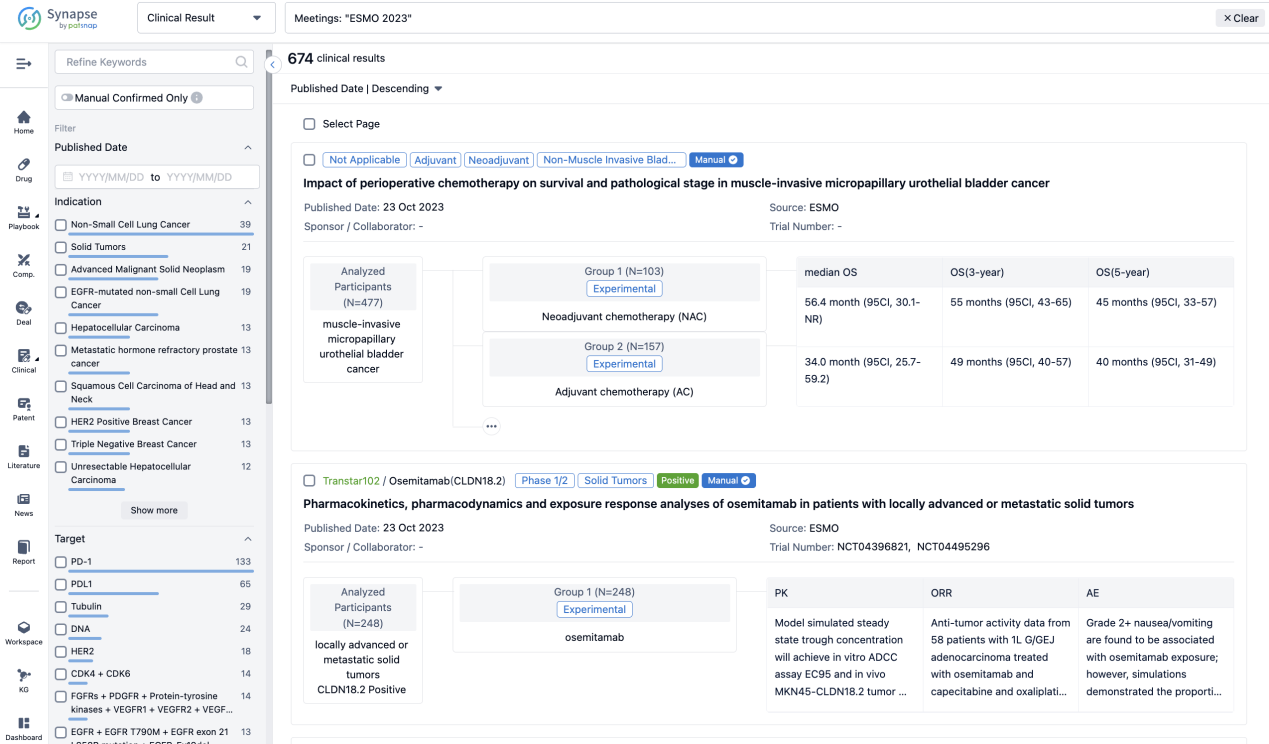
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
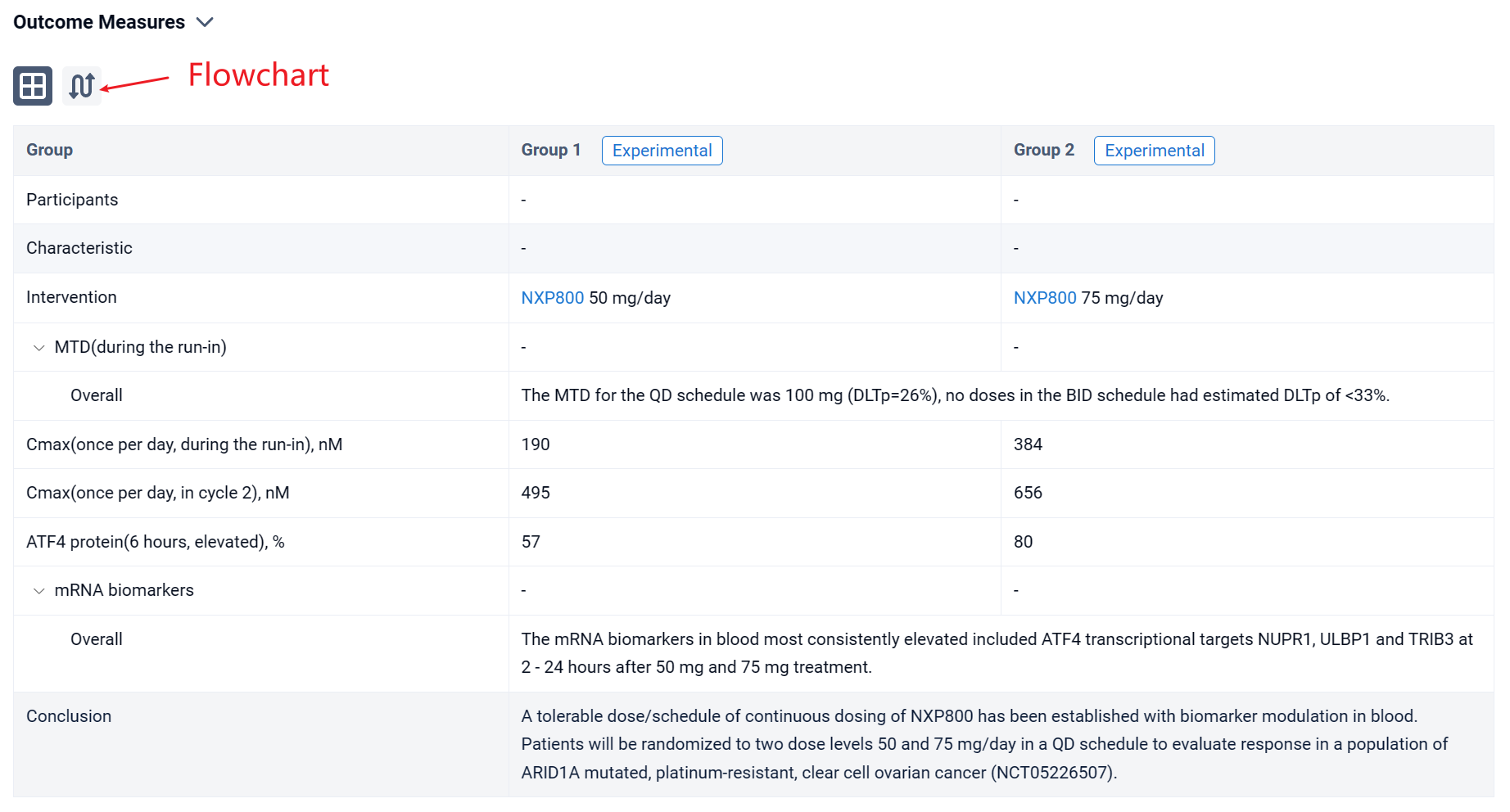
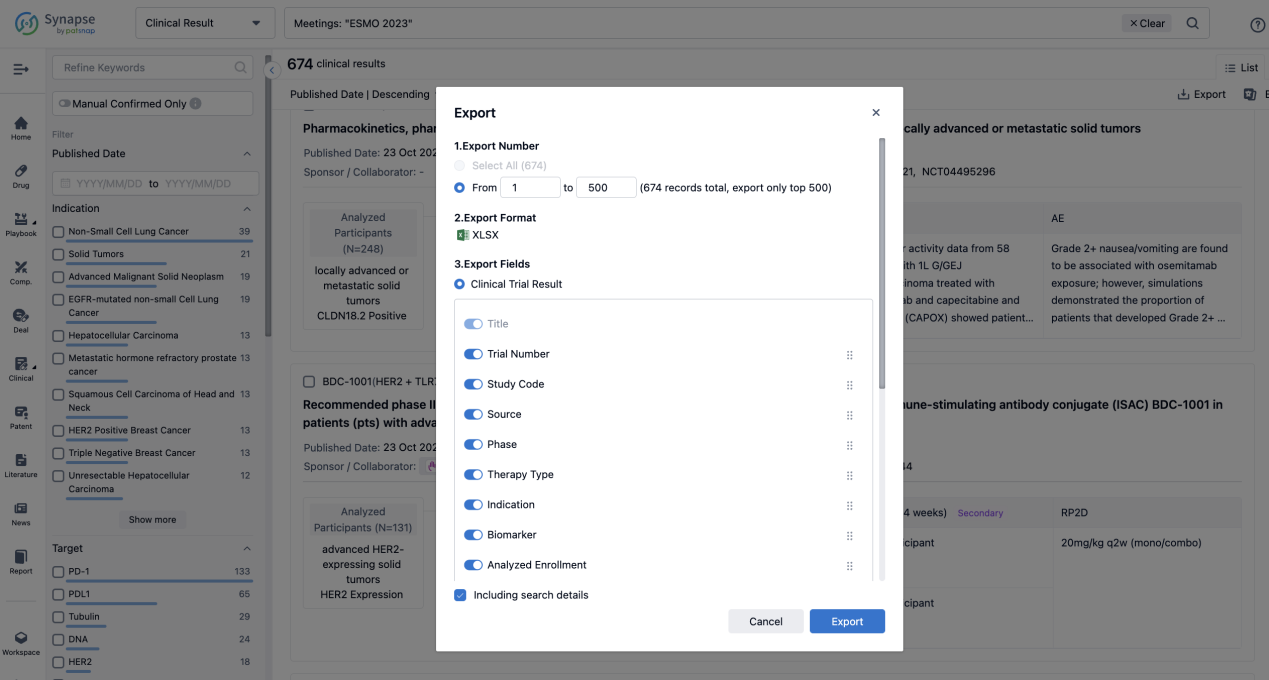
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!