Exploring Pirtobrutinib: Development, Approvals, and Patent Landscape
Pirtobrutinib is a small molecule drug developed by Redx Pharma Plc, targeting BTK C481S. Pirtobrutinib has received its first approval in the United States, with a global first approval date of January 2023. The highest phase of development for Pirtobrutinib is approved globally, and it is at the NDA/BLA stage in China. The drug is regulated under priority review, accelerated approval, and orphan drug status, indicating its potential to address unmet medical needs in the treatment of the aforementioned indications.
Below, we will use the drug Pirtobrutinib as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
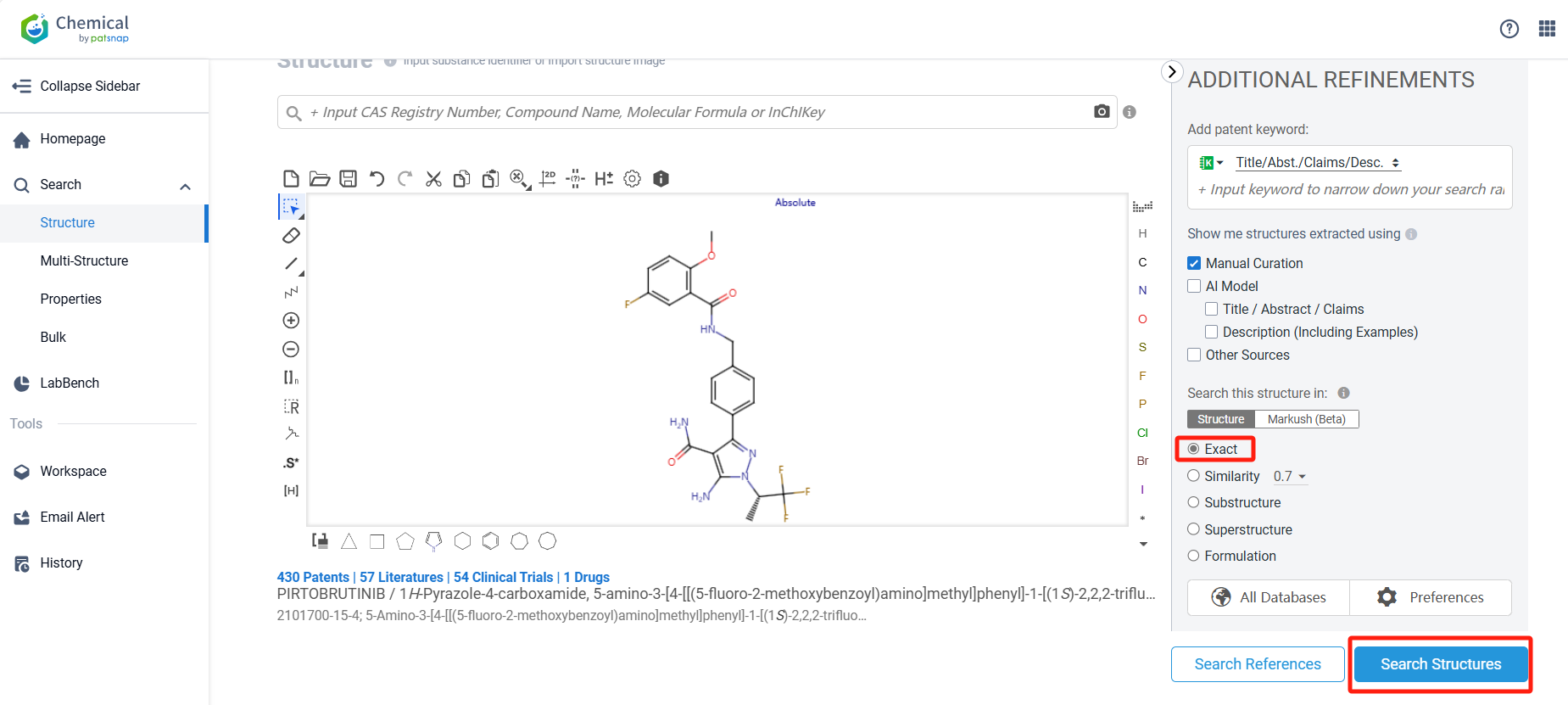
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Pirtobrutinib (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, we select "Exact Search", click on search structures, and you can find 48 patents. In the sidebar, select "Formulation" and "Use" under the "Claim Types" to search for patents related to new formulations and new indications. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
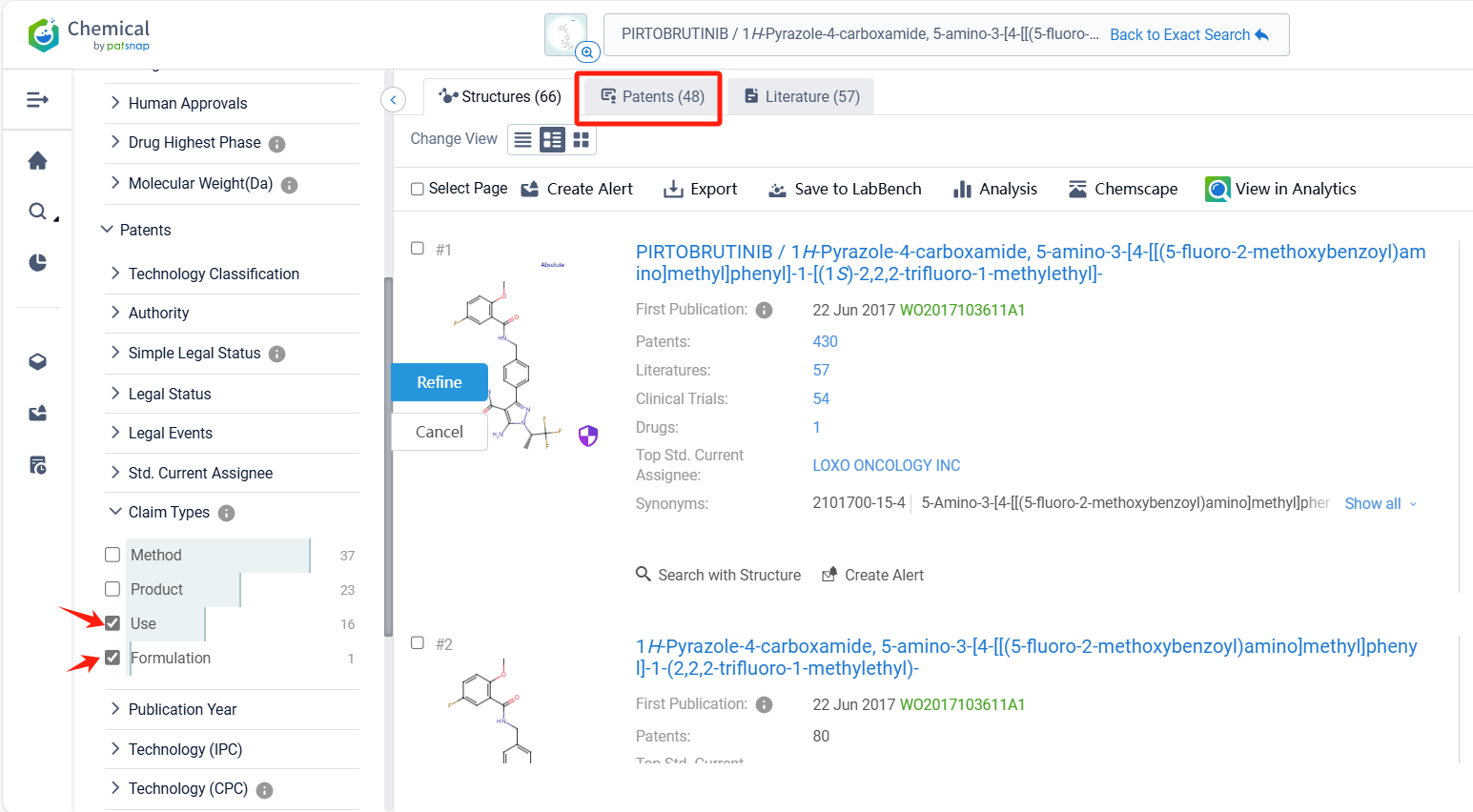
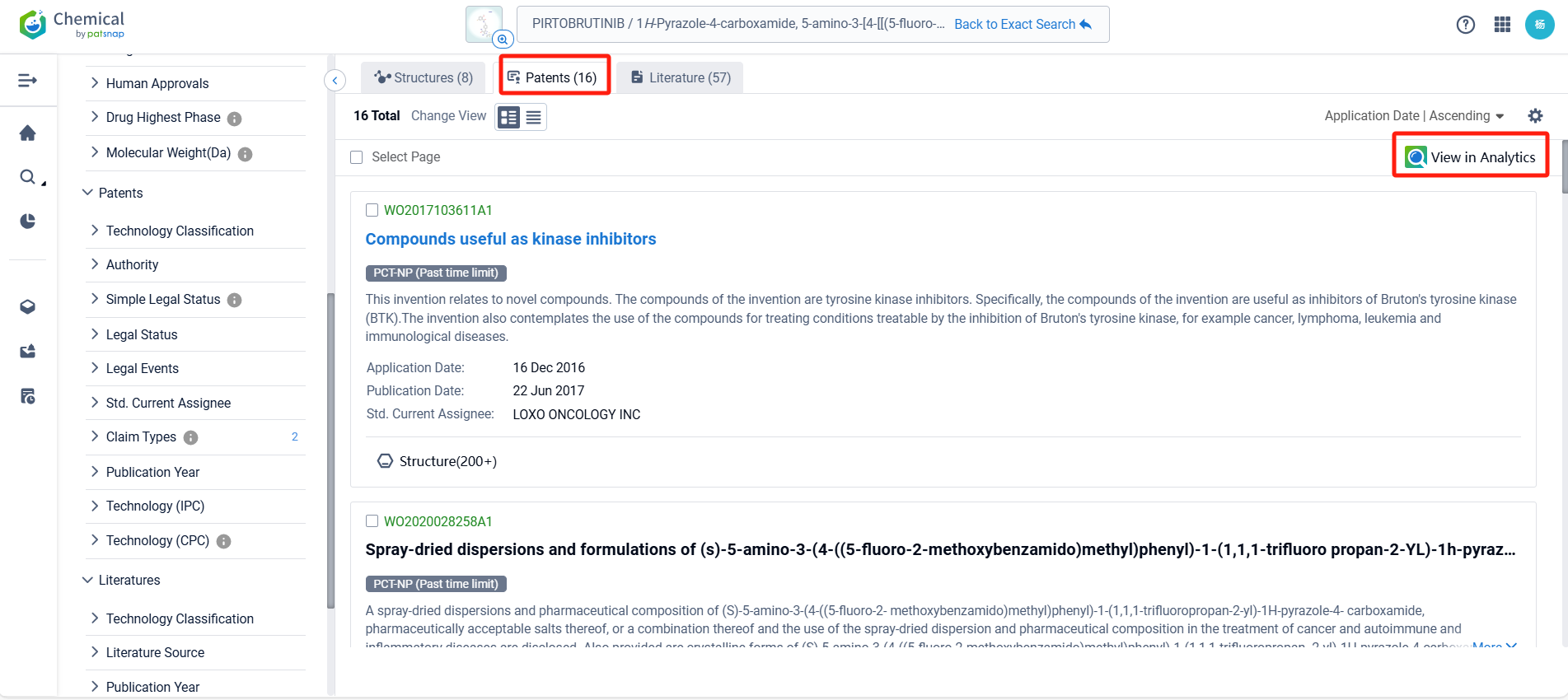
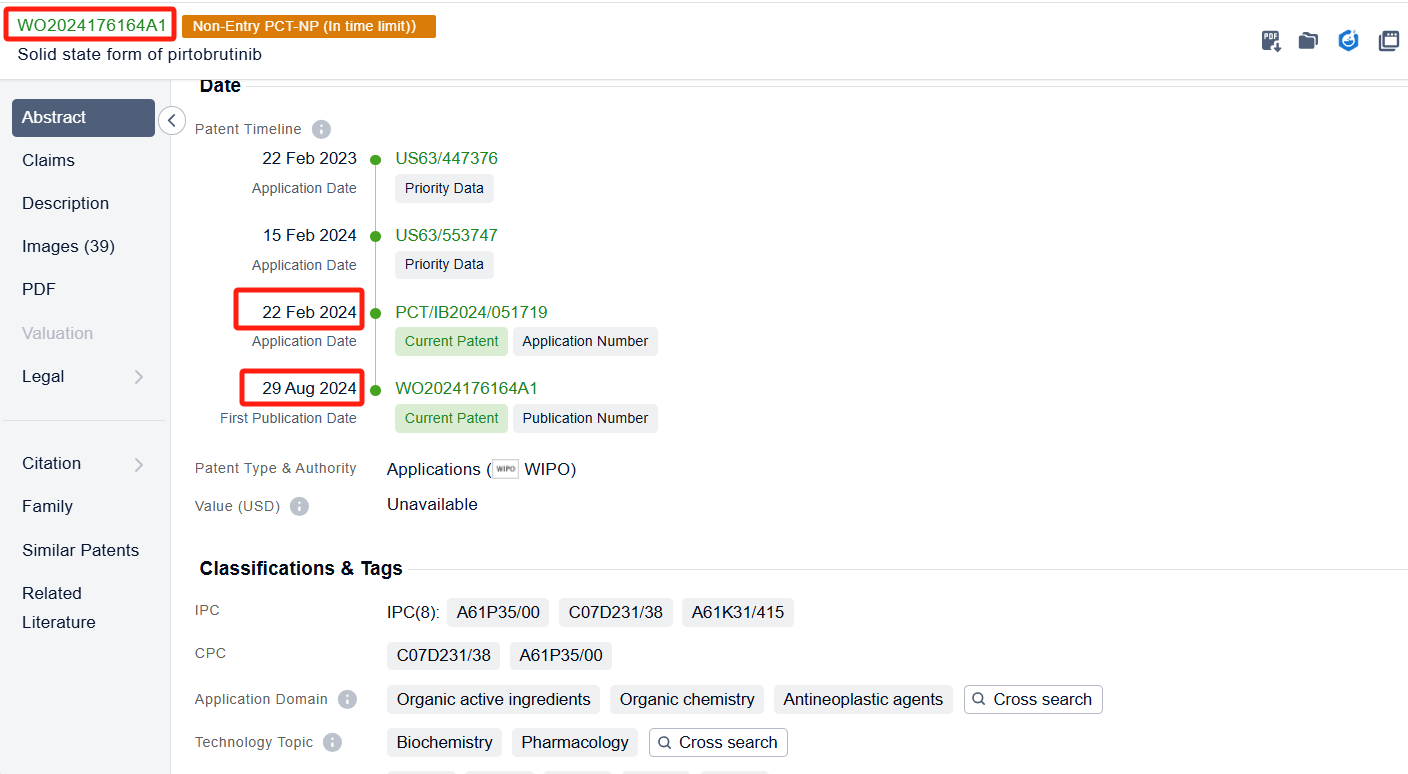
In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Pirtobrutinib. By reviewing the aforementioned patents, we can observe that LOXO ONCOLOGY, INC.'s international patent WO2020028258A1 (application date 20190729, publication date 20200206) discloses a spray-dried dispersions and formulations of Pirtobrutinib. The invention provides a pharmaceutical composition that includes a compound of Pirtobrutinib, an HPMCAS polymer, microcrystalline cellulose, mannitol, sodium starch glycolate, magnesium stearate, and silicon dioxide. The ratio of the compound of Formula I to the HPMCAS polymer is between about 1:4 and about 4:2. The composition has good flow properties and is suitable for tablet production. Its corresponding patents in Japan, South Korea, and the United States have all been granted.Additionally, ASSIA CHEMICAL INDUSTRIES LTD.'s patent WO2024176164A1 (application date 20240222, publication date 20240829) discloses a crystalline polymorph of Pirtobrutinib, which has various uses in the preparation of other solid state forms and salts/cocrystals thereof. 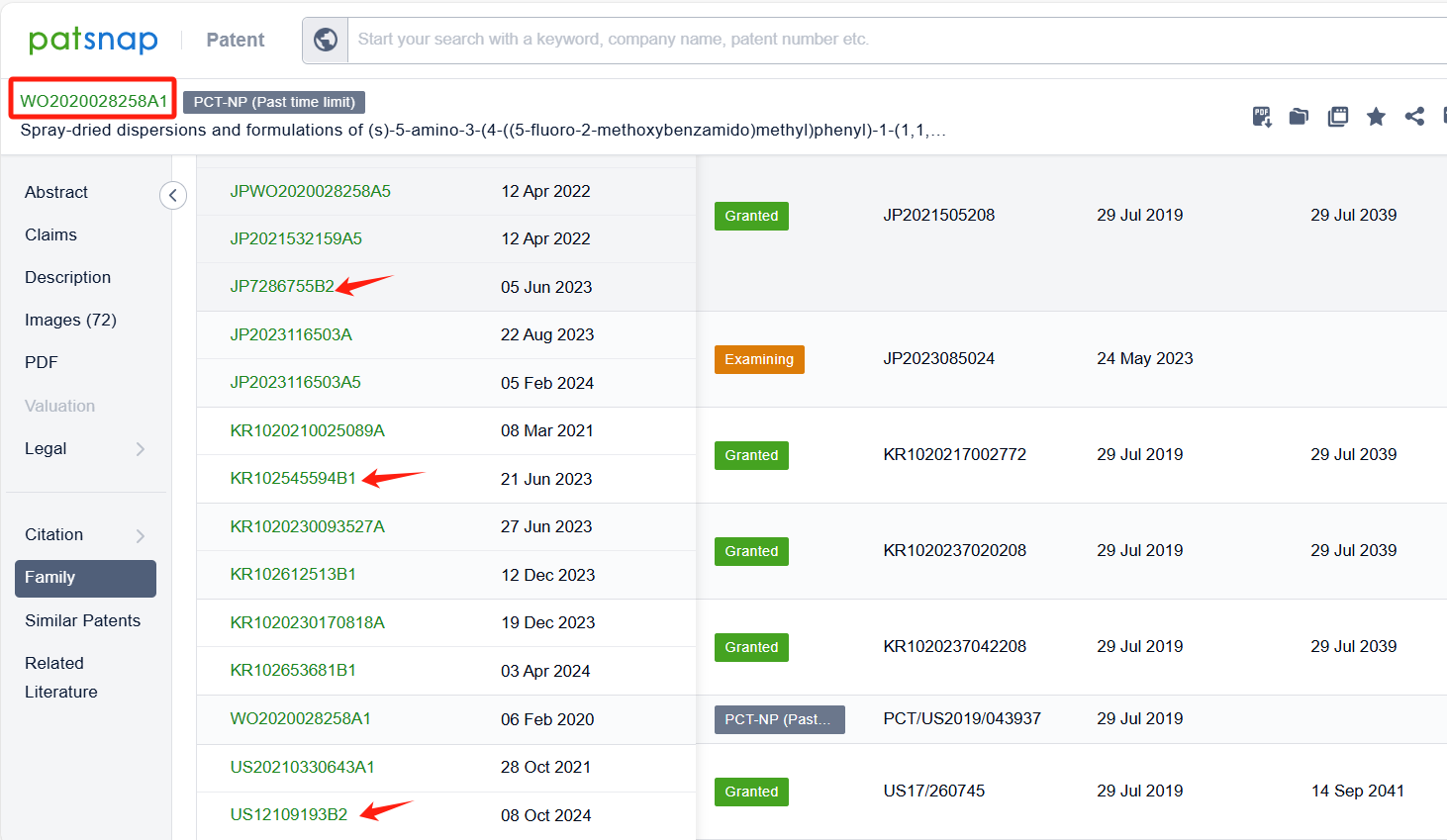
As a Business Development expert in the pharmaceutical industry, this information provides a comprehensive overview of Pirtobrutinib, its development stage, target indications, originator organization, and regulatory status. This data is crucial for evaluating the potential market opportunities and partnerships related to the drug, as well as understanding its competitive landscape and future commercial potential.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.