Soligenix starts a Phase 2 clinical study for SGX945 (dusquetide) to treat Behçet’s Disease
Soligenix, Inc. (Nasdaq: SNGX), a biopharmaceutical firm in the late phases of development, dedicated to the creation and commercialization of treatments for rare diseases with unmet medical needs, disclosed that it has commenced the enrollment of patients for its Phase 2 trial (protocol number DUS-AUBD-01) investigating SGX945 (dusquetide) for the management of Behçet’s Disease.
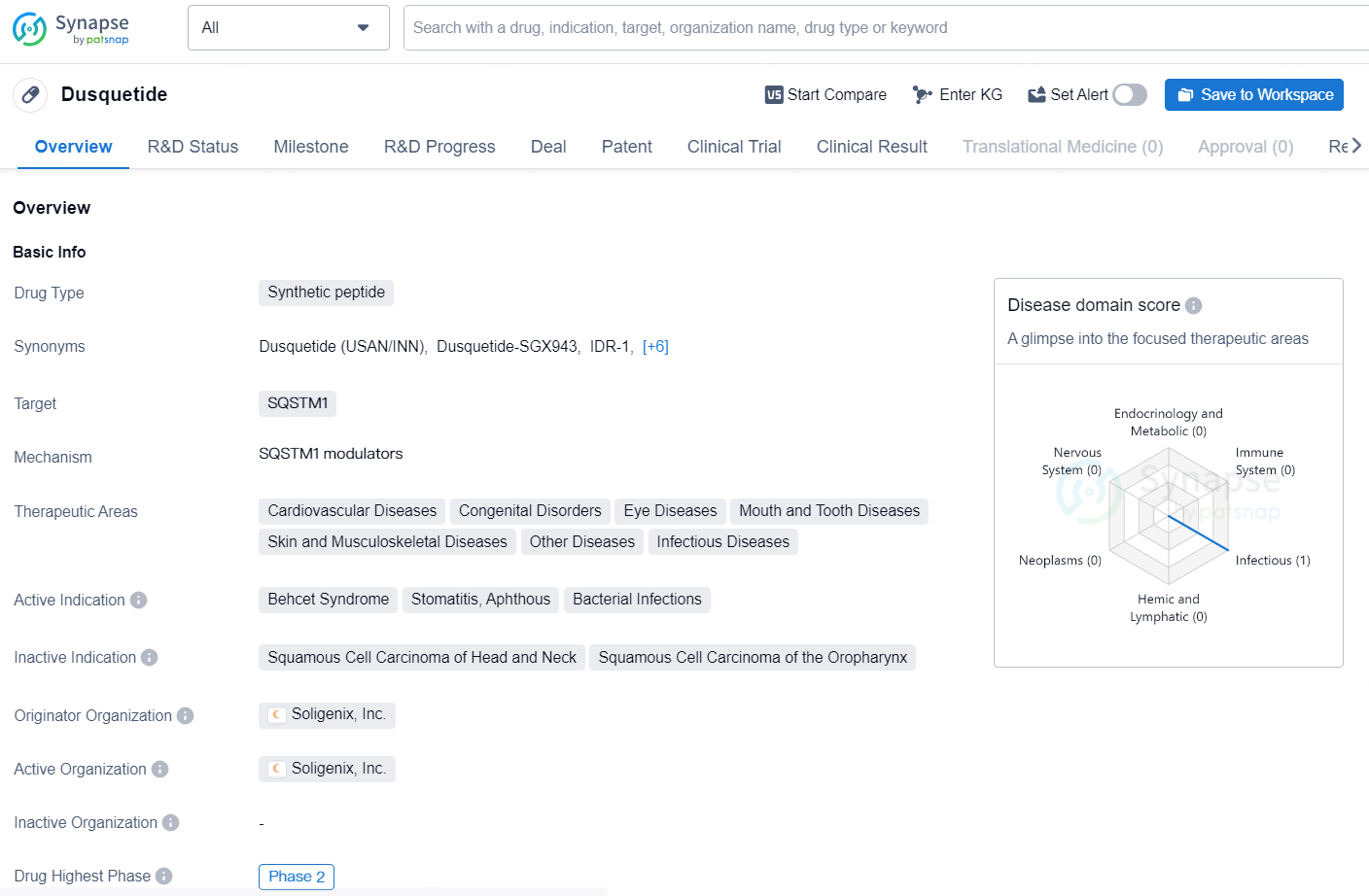
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“We are excited to announce that we have received clearance from the FDA and authorization from the Turkish Medicines and Medical Devices Agency to initiate patient recruitment for our SGX945 Phase 2a pilot study targeting aphthous ulcers associated with Behçet’s Disease,” said Christopher J. Schaber, PhD, President and CEO of Soligenix. “Our earlier research with dusquetide in treating oral mucositis has demonstrated its biological activity in aphthous ulcers resulting from chemotherapy and radiation. Considering the significant role of the innate immune system in the ulcers linked to Behçet’s Disease, combined with the pressing medical need for treatment options, particularly for more severe ulcers like those affecting the genital area and legs, we are optimistic that dusquetide could provide substantial relief for patients.
We are eager to broaden the application of dusquetide into various inflammatory conditions related to innate immunity, such as Behçet’s Disease, as part of our ongoing strategy to maximize the potential of this distinct compound. Behçet’s Disease represents a significant healthcare challenge, affecting up to 18,000 individuals in the U.S., 50,000 in Europe, 350,000 in Turkey, and potentially as many as 1 million globally. Given the encouraging biological activity observed in aphthous ulcers in oral mucositis cases, we are hopeful that dusquetide will serve as an important treatment option for underserved patients dealing with this challenging chronic autoimmune condition. We anticipate completing patient recruitment and sharing study findings by the first half of 2025.”
The pilot clinical trial for SGX945 will be an open-label investigation enrolling around 25 participants aged 18 and older who are experiencing mild to moderate Behçet’s Disease with active oral and/or genital ulcers. Subjects will receive SGX945 through a twice-weekly intravenous (IV) infusion lasting 4 minutes over a period of 4 weeks, followed by an additional 4 weeks for monitoring. Efficacy measures will include the degree of lesion resolution, time taken for lesion resolution, and patient-reported quality of life evaluations.
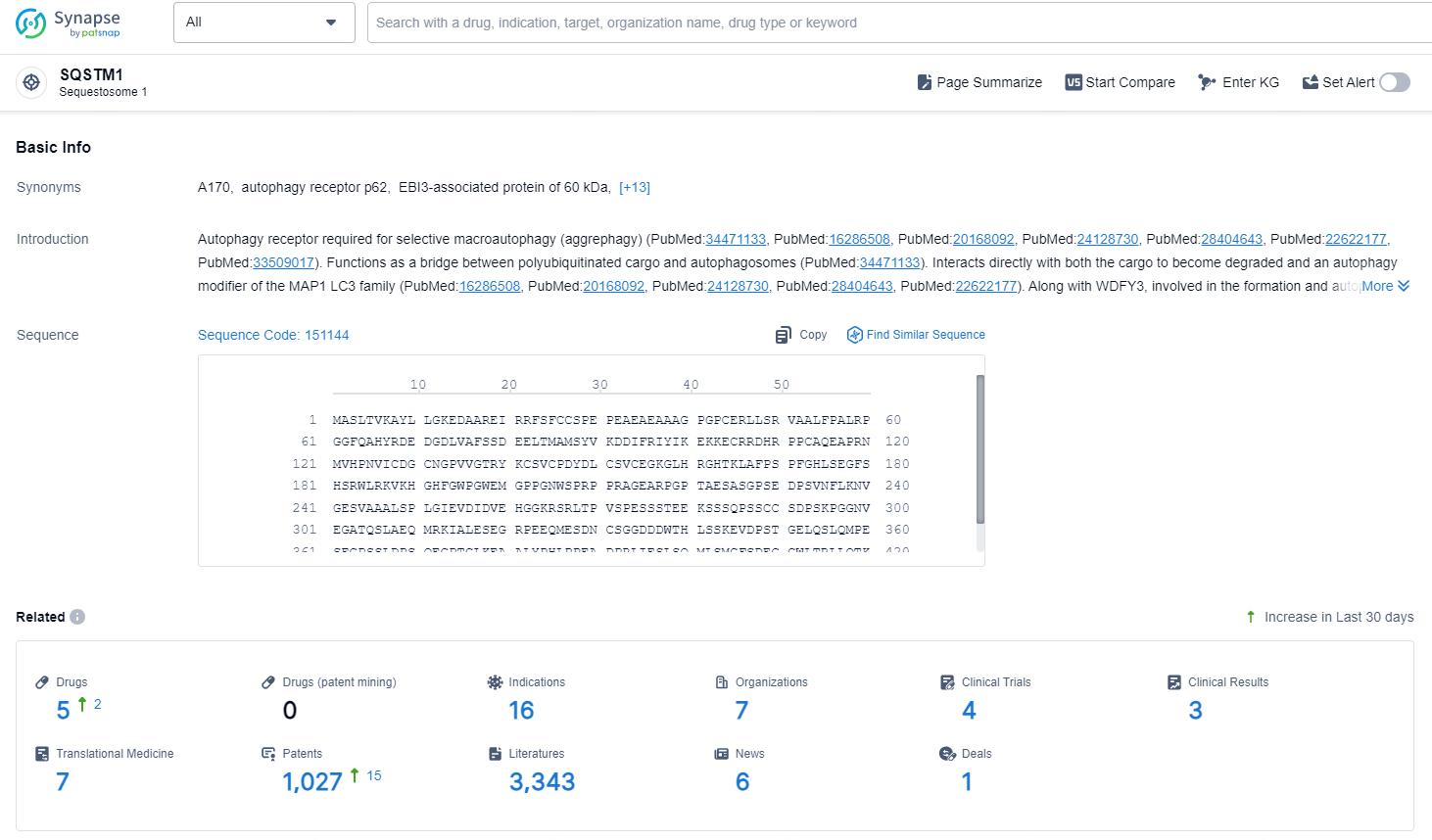
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 18, 2024, there are 5 investigational drugs for the SQSTM1 target, including 16 indications, 7 R&D institutions involved, with related clinical trials reaching 4, and as many as 1027 patents.
Dusquetide is a synthetic peptide drug developed by Soligenix, Inc., targeting the SQSTM1 protein. The drug is indicated for the treatment of a wide range of therapeutic areas including cardiovascular diseases, congenital disorders, eye diseases, mouth and tooth diseases, skin and musculoskeletal diseases, as well as other infectious diseases.