Merck Secures Global Rights to LM-299, a PD-1/VEGF Targeting Antibody
Merck (NYSE: MRK), referred to as MSD in regions outside the United States and Canada, along with LaNova Medicines Ltd. (LaNova), a privately-owned biotech firm in the clinical development phase, has announced that Merck has secured an exclusive worldwide license for the development, production, and commercialization of LM-299. This is a new investigational bispecific antibody targeting PD-1/VEGF from LaNova.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“Merck is dedicated to building a robust and varied oncology pipeline that encompasses distinct mechanisms and multiple modalities,” stated Dr. Dean Y. Li, president of Merck Research Laboratories. “This partnership enhances Merck’s expanding oncology portfolio, and we are eager to expedite the development of LM-299 for patients in need.”
According to the agreement, LaNova has provided Merck with an exclusive global license for the development, manufacturing, and commercialization of LM-299. LaNova will receive an initial payment of $588 million and could receive up to $2.7 billion in milestone payments tied to the technology transfer, development, regulatory approval, and marketing of LM-299 for various applications.
“This partnership with Merck highlights the dedication of LaNova's skilled scientific team behind the creation of LM-299,” remarked Dr. Crystal Qin, founder, chairwoman, and CEO of LaNova. “Through innovative internal research and strategic external collaborations, LaNova is focused on advancing its pipeline to serve patients globally.”
The finalization of this transaction is pending approval under the Hart-Scott-Rodino Antitrust Improvements Act and other standard conditions. The deal is anticipated to close in the fourth quarter of 2024. Merck plans to recognize a pre-tax expense related to the $588 million payment upon closing, which will be reflected in both GAAP and non-GAAP results for the period in which the transaction concludes, with the EPS effect of this charge to be revealed at that time.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
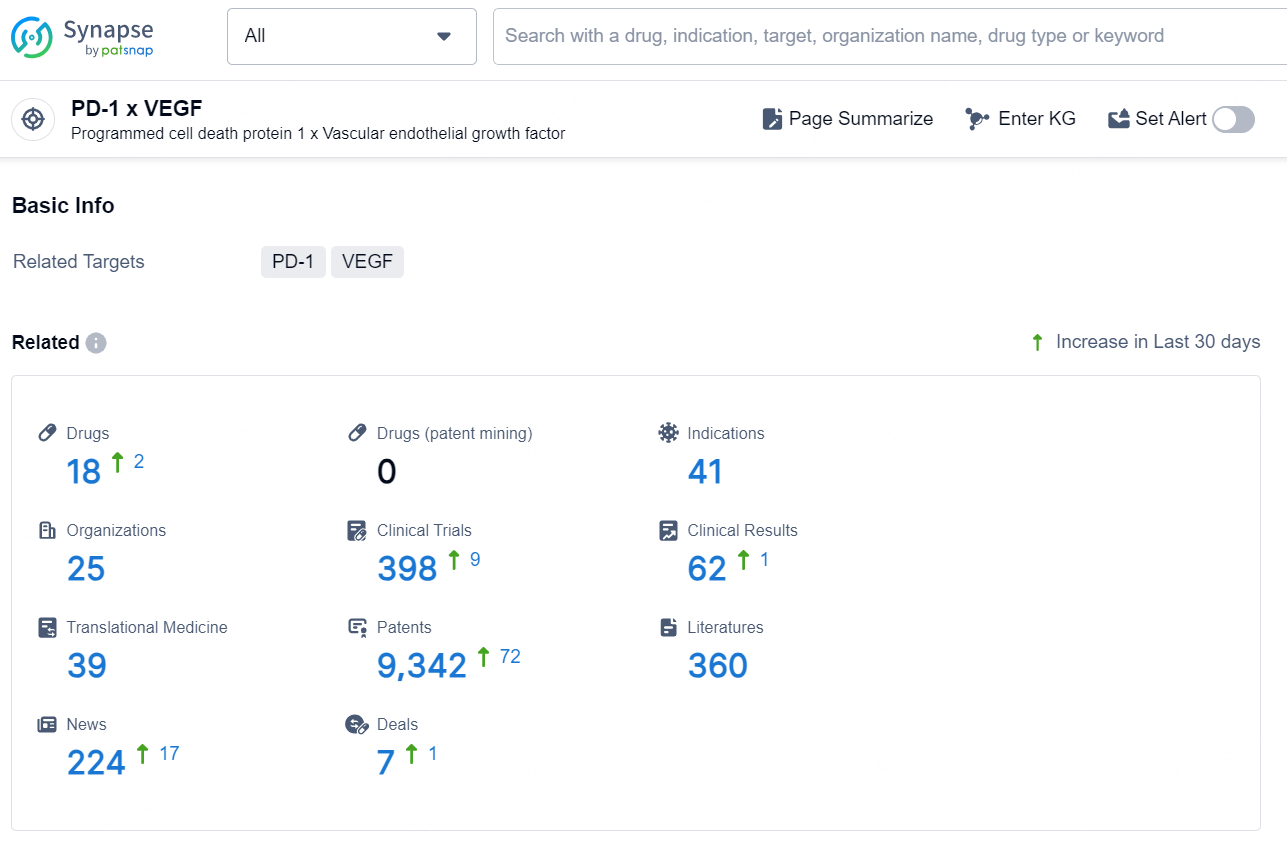
According to the data provided by the Synapse Database, As of November 18, 2024, there are 18 investigational drugs for the PD-1 and VEGF target, including 41 indications, 25 R&D institutions involved, with related clinical trials reaching 398, and as many as 9342 patents.
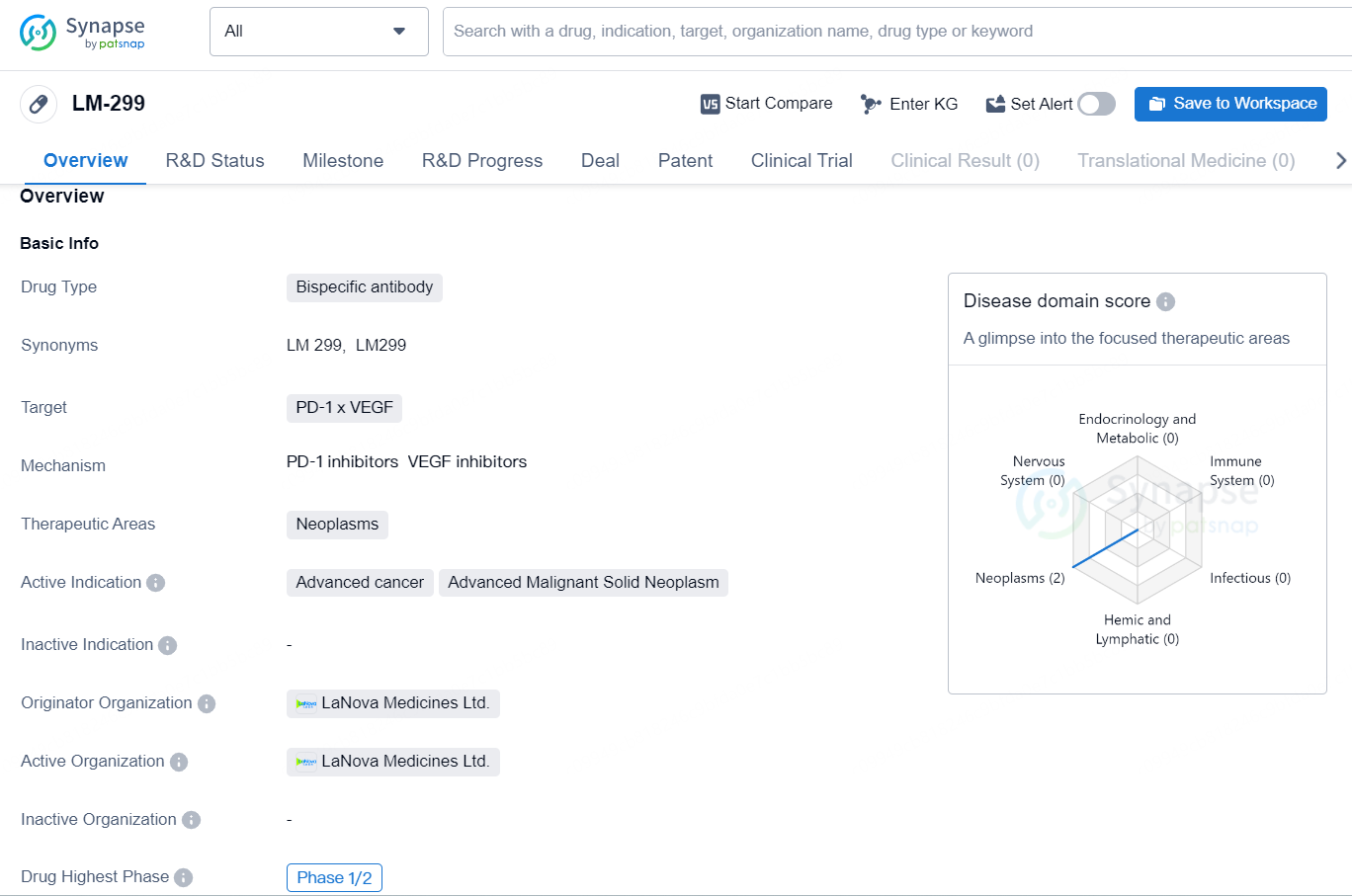
LM-299 is a bispecific antibody drug developed by LaNova Medicines Ltd., which targets the PD-1 and VEGF proteins. This drug falls into the therapeutic area of neoplasms, specifically for the treatment of advanced cancer and advanced malignant solid neoplasms. The highest phase of development for LM-299 is currently Phase 1/2 globally, with the same phase of development in China.