Exploring Rifabutin's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Rifabutin's R&D Progress
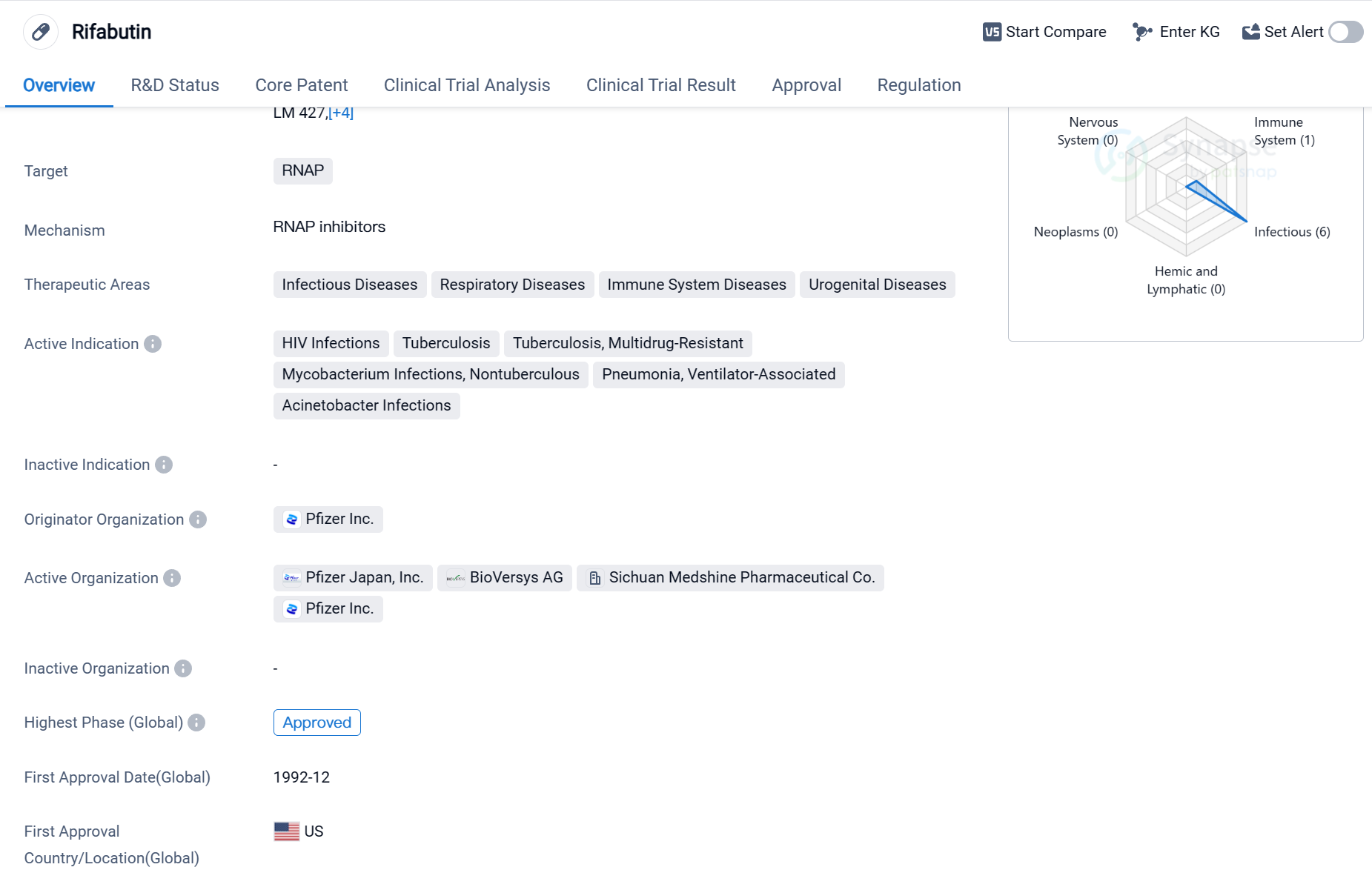
Rifabutin is a small molecule drug that targets RNAP (RNA polymerase), making it a potential treatment option for various infectious diseases, respiratory diseases, immune system diseases, and urogenital diseases. It has been approved for use in multiple indications, including HIV infections, tuberculosis (both drug-sensitive and multidrug-resistant), nontuberculous mycobacterium infections, ventilator-associated pneumonia, and Acinetobacter infections.
The drug was developed by Pfizer Inc., a renowned pharmaceutical company. Rifabutin received its first approval in the United States in December 1992, making it a well-established medication in the global market. It has also obtained approval in China, indicating its potential to address medical needs in multiple countries.
Rifabutin's approval as an orphan drug suggests that it is intended to treat rare diseases or conditions that affect a small number of patients. This regulatory designation often provides incentives for pharmaceutical companies to develop drugs for such conditions.
As a small molecule drug, Rifabutin is likely to have a well-defined chemical structure, allowing for easier synthesis and formulation. This characteristic can contribute to its stability, bioavailability, and potential for oral administration, which are crucial factors in drug development.
Given its broad therapeutic areas, Rifabutin has the potential to address significant unmet medical needs. Infectious diseases, respiratory diseases, immune system diseases, and urogenital diseases are prevalent worldwide, affecting millions of individuals. The drug's approval for HIV infections and tuberculosis, including multidrug-resistant strains, highlights its potential in combating these challenging conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Rifabutin: RNAP inhibitors
RNAP inhibitors are a type of drugs that specifically target and inhibit the activity of RNA polymerase (RNAP), an enzyme responsible for synthesizing RNA from DNA templates. In the context of biomedicine, RNAP inhibitors are of significant interest due to their potential therapeutic applications in treating various diseases caused by RNA viruses, such as influenza, hepatitis C, and COVID-19.
By inhibiting RNAP, these drugs prevent the viral RNA replication and transcription processes necessary for the virus to replicate and spread within the host. This inhibition can effectively suppress viral replication, reduce viral load, and potentially alleviate the severity of the viral infection.
RNAP inhibitors can be developed through different mechanisms, including direct binding to the enzyme's active site or interfering with the initiation or elongation steps of RNA synthesis. Some examples of RNAP inhibitors include remdesivir, which has been authorized for emergency use in treating COVID-19, and rifampin, a broad-spectrum antibiotic that inhibits bacterial RNAP.
It's worth noting that RNAP inhibitors can have off-target effects, meaning they may also affect the activity of human RNA polymerases. This can lead to potential side effects and requires careful evaluation during drug development. Nonetheless, RNAP inhibitors hold promise as a targeted therapeutic approach against RNA viruses and continue to be an active area of research in biomedicine.
Drug Target R&D Trends for Rifabutin
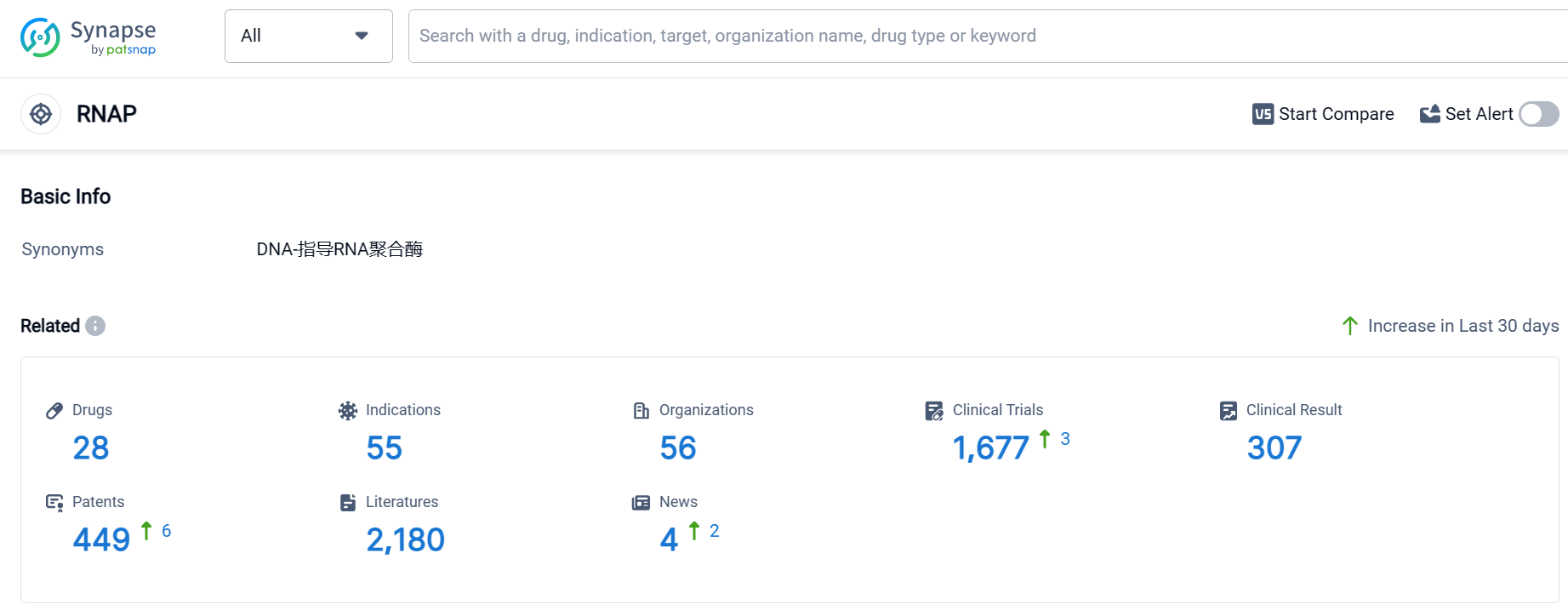
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 28 RNAP drugs worldwide, from 56 organizations, covering 55 indications, and conducting 1677 clinical trials.
The analysis of the target RNAP reveals a competitive landscape with multiple companies focusing on its development. Merck & Co., Inc., Roche Holding AG, Chengdu First Pharmaceutical Co. Ltd., and RedHill Biopharma Ltd. are among the companies growing fastest under the target RNAP. The highest stage of development for the target RNAP is the approved stage, with small molecule drugs being the most prominent drug type. The indications with the highest number of approved drugs include Tuberculosis, Hepatitis C, and Hepatitis C, Chronic. The United States, China, and Japan are the countries/locations developing fastest under the target RNAP. Overall, the analysis suggests a strong R&D focus on the target RNAP, with significant progress in developing treatments for various indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Rifabutin is a small molecule drug developed by Pfizer Inc. that targets RNAP. It has been approved for various indications, including HIV infections, tuberculosis, pneumonia, and Acinetobacter infections. With its first approval dating back to 1992 in the United States, Rifabutin has established itself as a reliable treatment option. Its orphan drug status further emphasizes its potential to address unmet medical needs. As a result, Rifabutin holds promise in the field of biomedicine and may contribute significantly to the treatment of infectious, respiratory, immune system, and urogenital diseases.