Exploring the Latest dual-mechanism therapy Deal by WindtreeTherapeutics: A Guide to Rapidly Accessing Transaction Insights
On January 17, 2024, Windtree Therapeutics, Inc. announced that it had entered into a licensing agreement with Lee's Pharmaceutical Holdings Ltd. (Lee's Pharm) for the development and commercialization of Windtree's candidate product istaroxime for the treatment of acute heart failure and cardiogenic shock in the Greater China region. Additionally, the agreement permits Windtree to develop a preclinical next-generation SERCA2a activator (dual mechanism SERCA2a activator) and rostafuroxin, a Phase 2 candidate for treating certain genotypes of hypertension. Under the terms of the agreement, Lee's Pharm will obtain the license to develop and commercialize istaroxime, the dual mechanism SERCA2a activator, and rostafuroxin within the Greater China region. Upon the achievement of certain milestones, Windtree will be entitled to receive payments up to $138.1 million, plus royalties up to a low double-digit percentage. Lee's Pharm will be responsible for the development, production, regulatory, and commercial costs of the covered products within the licensed territory, as well as a portion of the global clinical trials conducted in the authorized regions. The agreement also establishes a joint steering committee and a joint development committee to oversee the development activities in the region, with Windtree retaining the final decision-making authority over clinical plans, and a joint commercialization committee. With this partnership, Lee's Pharm has nearly brought the entire R&D pipeline of Windtree into China.
About Istaroxime
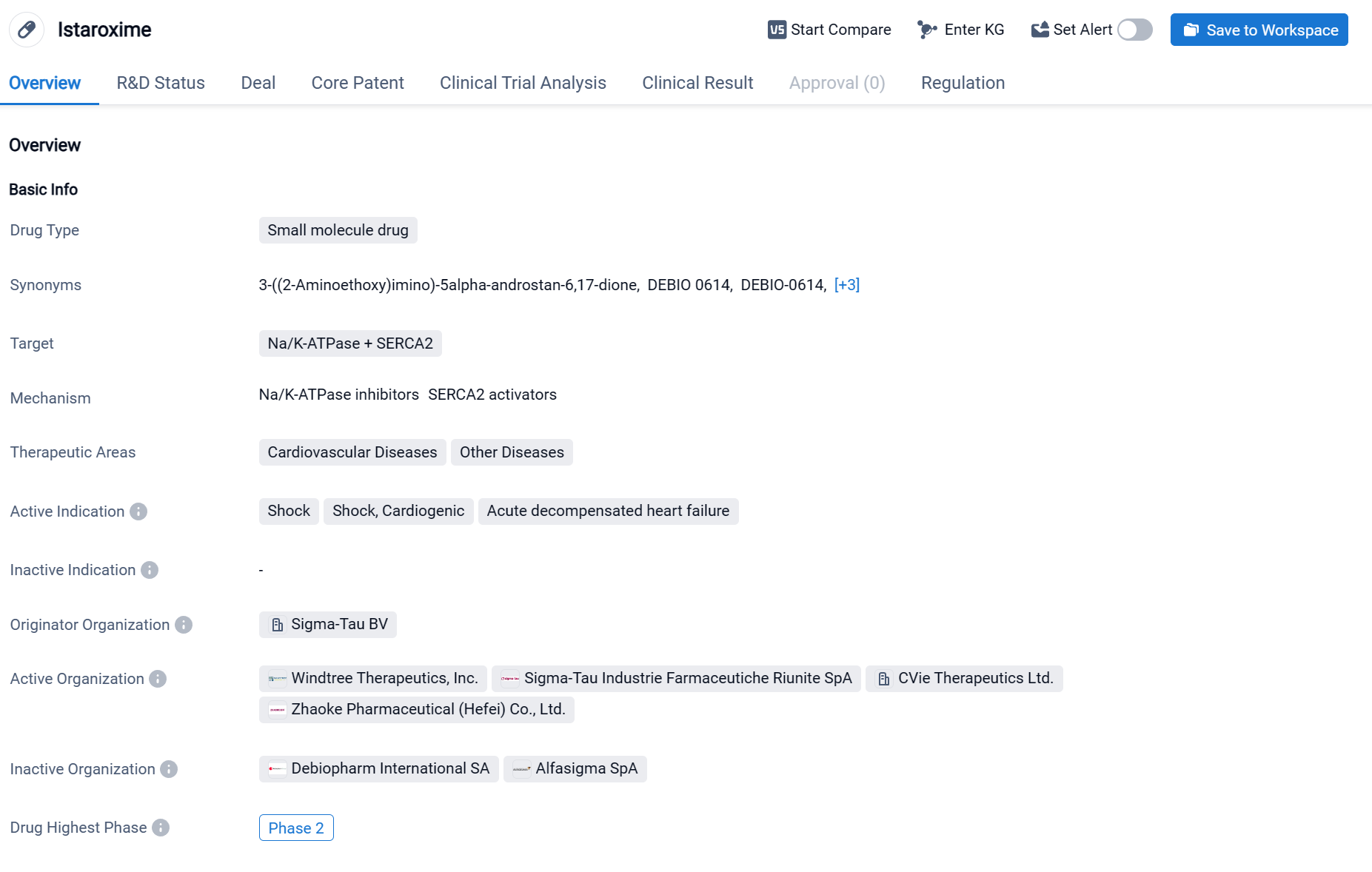
Istaroxime is a small molecule drug that targets Na/K-ATPase and SERCA2. It falls under the therapeutic areas of cardiovascular diseases and other diseases. The drug is primarily indicated for shock, cardiogenic shock, and acute decompensated heart failure. It is developed by Sigma-Tau BV, an originator organization in the pharmaceutical industry. Click the image below to directly embark on the exploration journey with the Istaroxime!
The study NCT04325035, published in the Journal of Clinical Medicine, aimed to compare the effects of Istaroxime with placebo in patients with cardiogenic shock associated with acute heart failure (AHF) presenting with persistent low blood pressure (systolic blood pressure, SBP, between 75 mmHg and 90 mmHg) without clinical signs of low perfusion. Patients were randomly assigned to receive 1.0 μg/(kg·min), 1.5 μg/(kg·min) of Istaroxime, or placebo, with a continuous infusion of Istaroxime or placebo for 24 hours.
The clinical results indicated that:
Compared with placebo, patients treated with Istaroxime had higher systolic blood pressure (SBP) from baseline to 24 hours, with increases also observed in diastolic blood pressure (DBP) and mean arterial pressure (MAP). Istaroxime treatment was associated with more adverse events, such as nausea, vomiting, and infusion site pain, compared with placebo, but not with arrhythmias or worsening renal function.
The study demonstrated that Istaroxime increased SBP and improved echocardiographic parameters (increased cardiac index, decreased left atrial and left ventricular size).
Preclinical trial data indicate that RBD7022 has favorable safety characteristics and a potent lipid-lowering effect. The drug's lipid-lowering action is characterized by stable levels of lipid reduction and durable efficacy, with a single administration sustaining efficacy for several months and achieving an inhibition of LDL-C by over 50%.
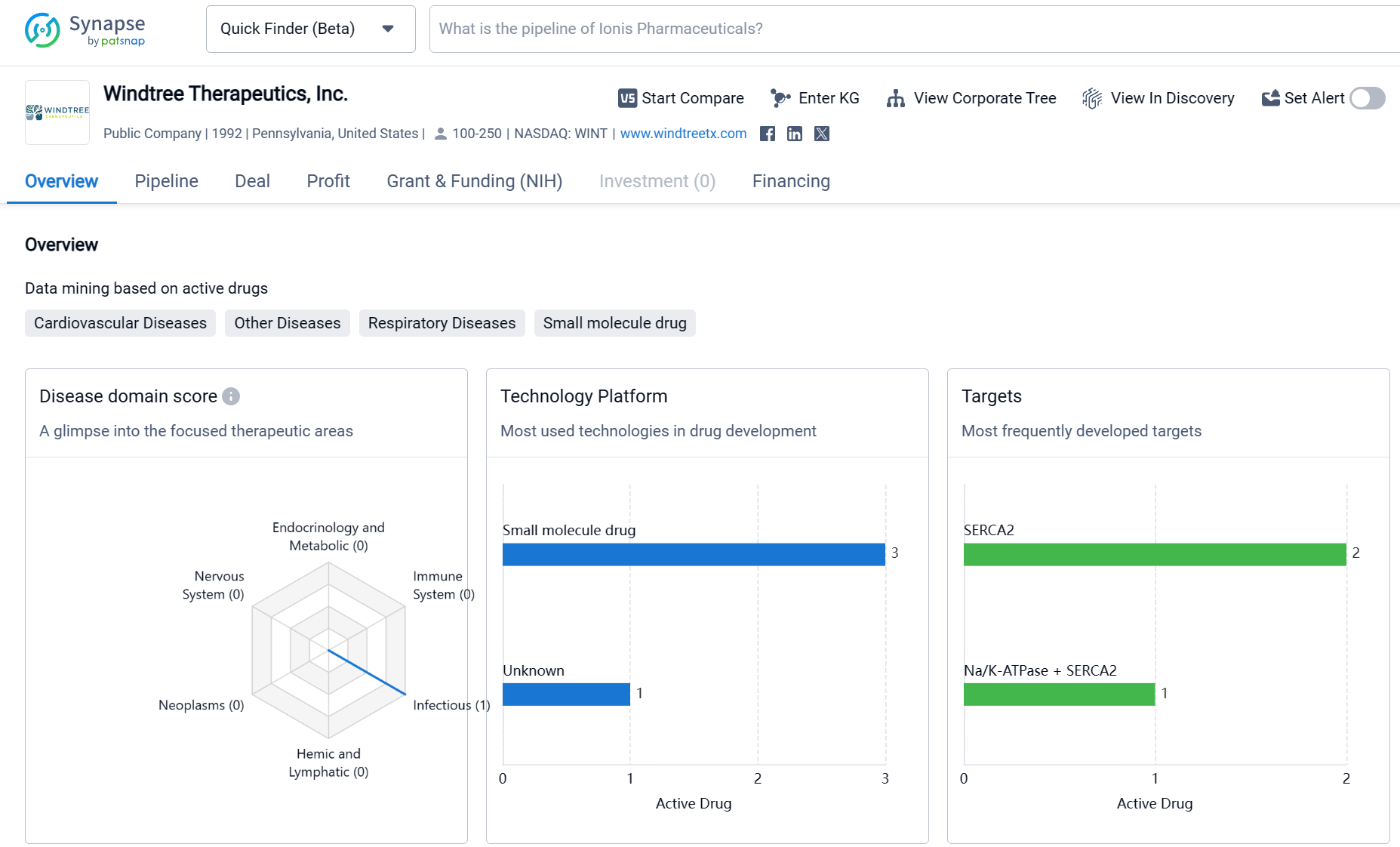
About Windtree Therapeutics
Windtree Therapeutics, Inc. is a biopharmaceutical company that has been active in the field of drug development since 1992. The company has a diverse portfolio of drugs targeting various therapeutic areas, with a particular focus on cardiovascular diseases. While the company has made progress in the development of drugs, with two drugs in phase 2 trials, it has not yet reached the stage of seeking regulatory approval for any of its drugs. The pipeline also indicates that the company has several drugs in the early stages of development or evaluation.
Windtree is advancing late-stage interventions for cardiovascular diseases to treat patients in critical conditions. Utilizing novel scientific and clinical approaches, Windtree is developing a diverse pipeline centered on compounds capable of activating SERCA2a.
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
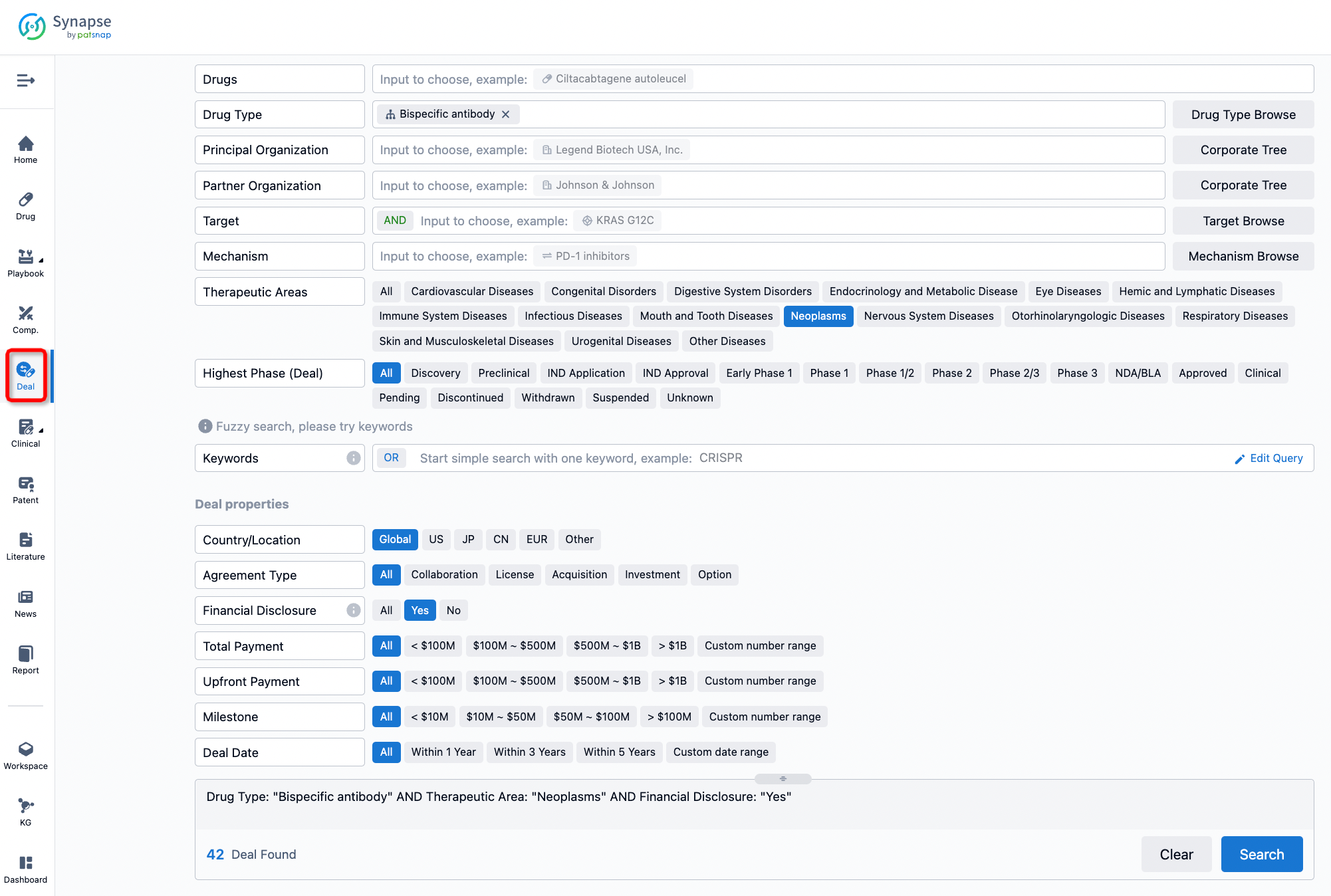
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
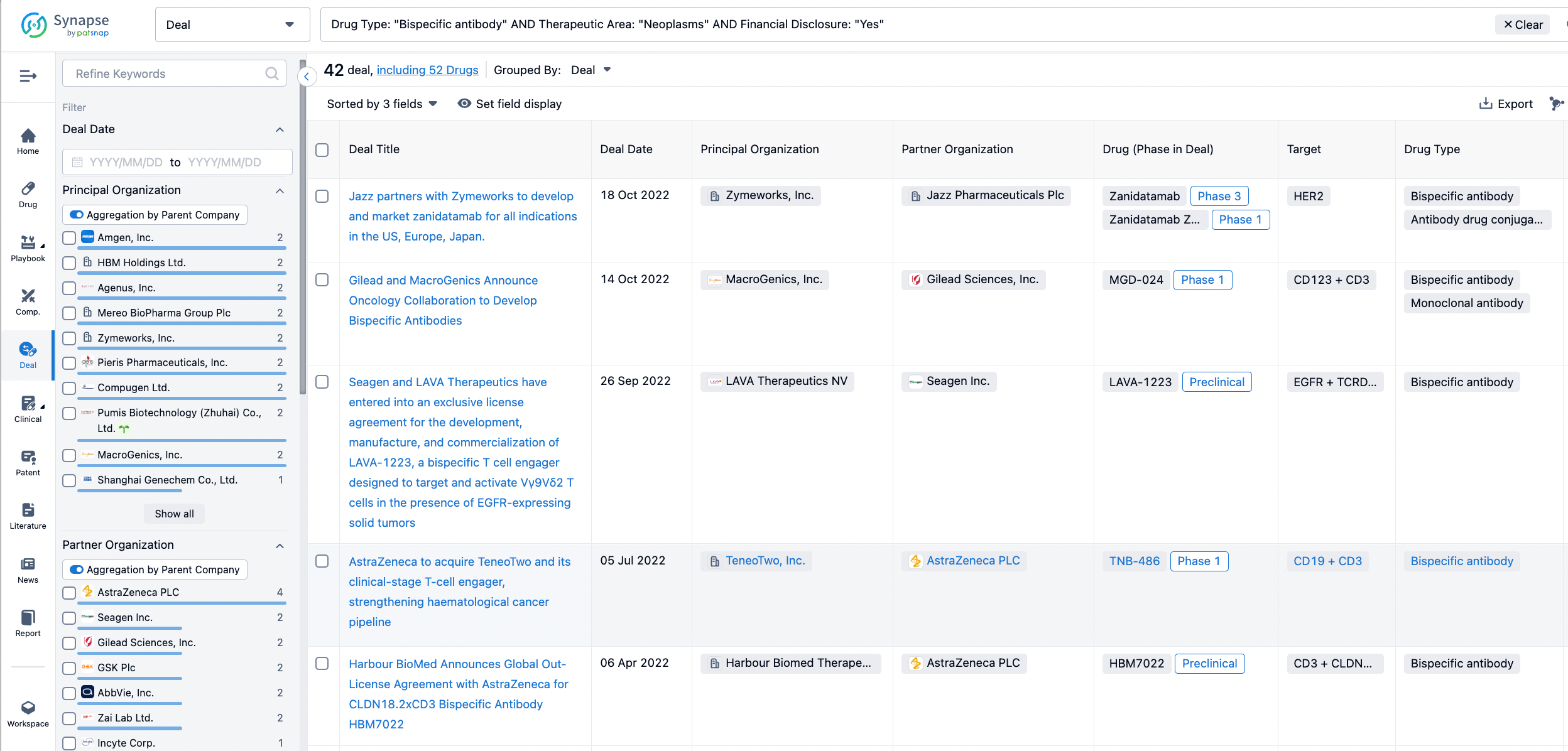
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
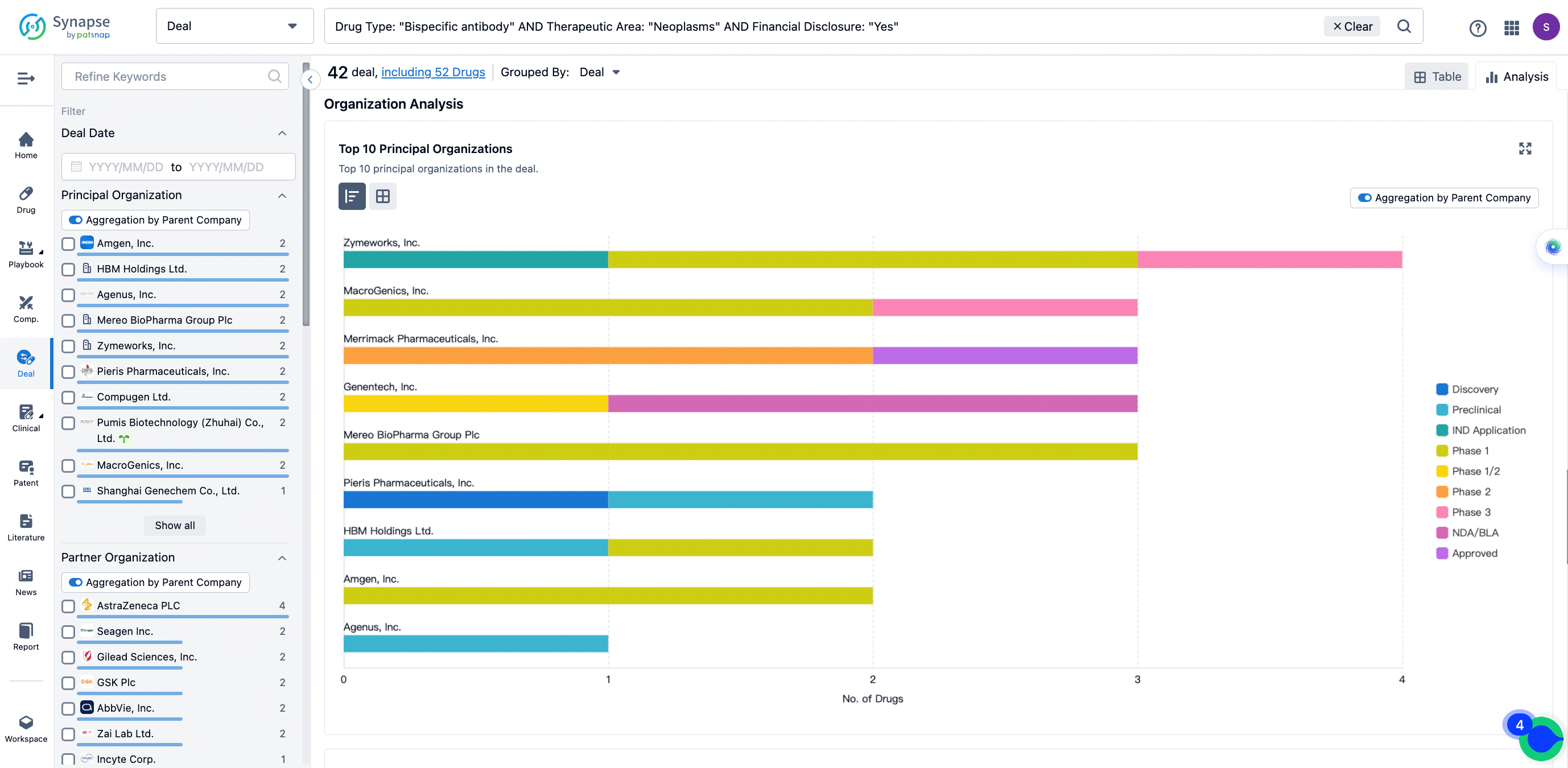
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
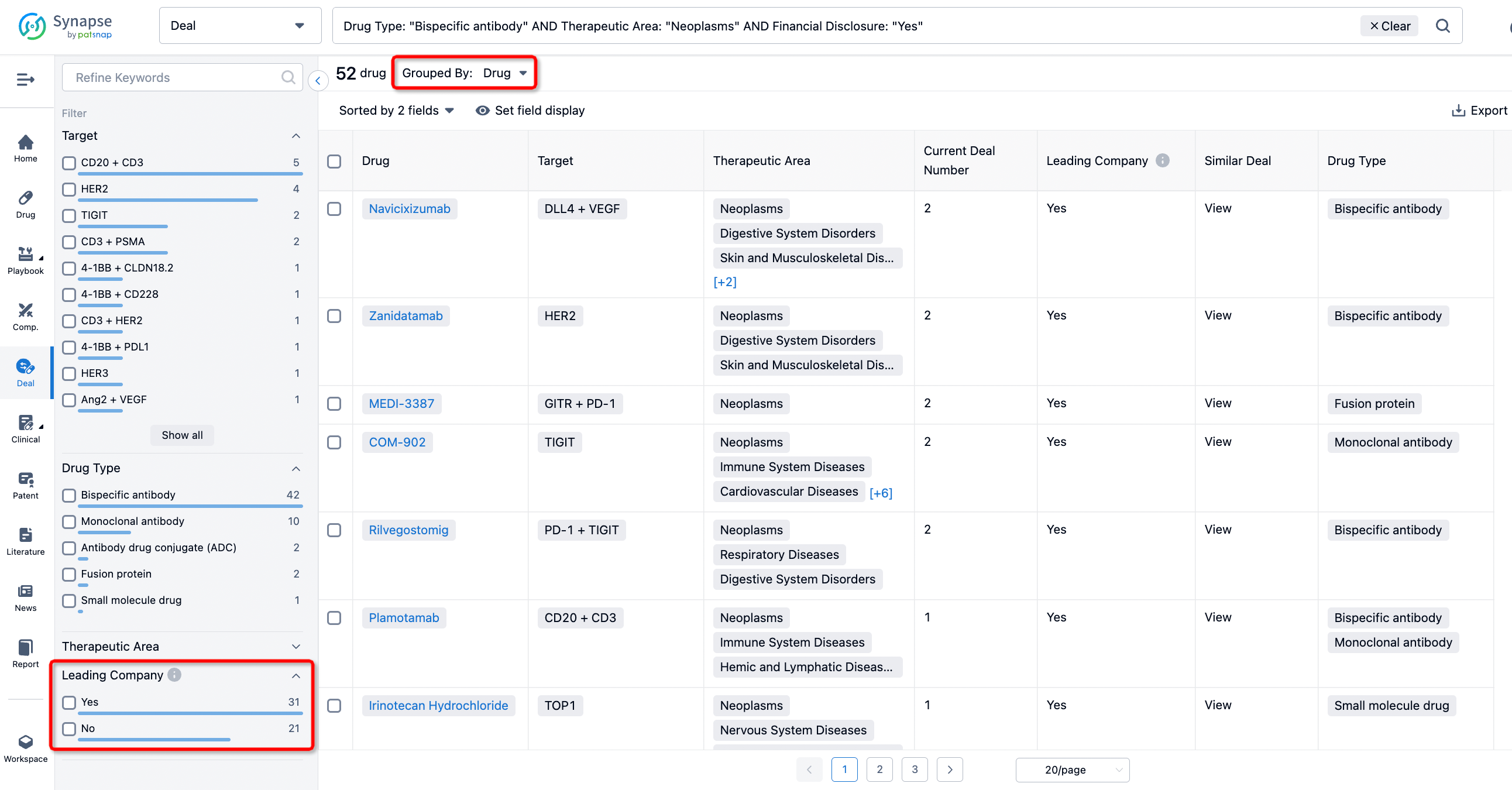
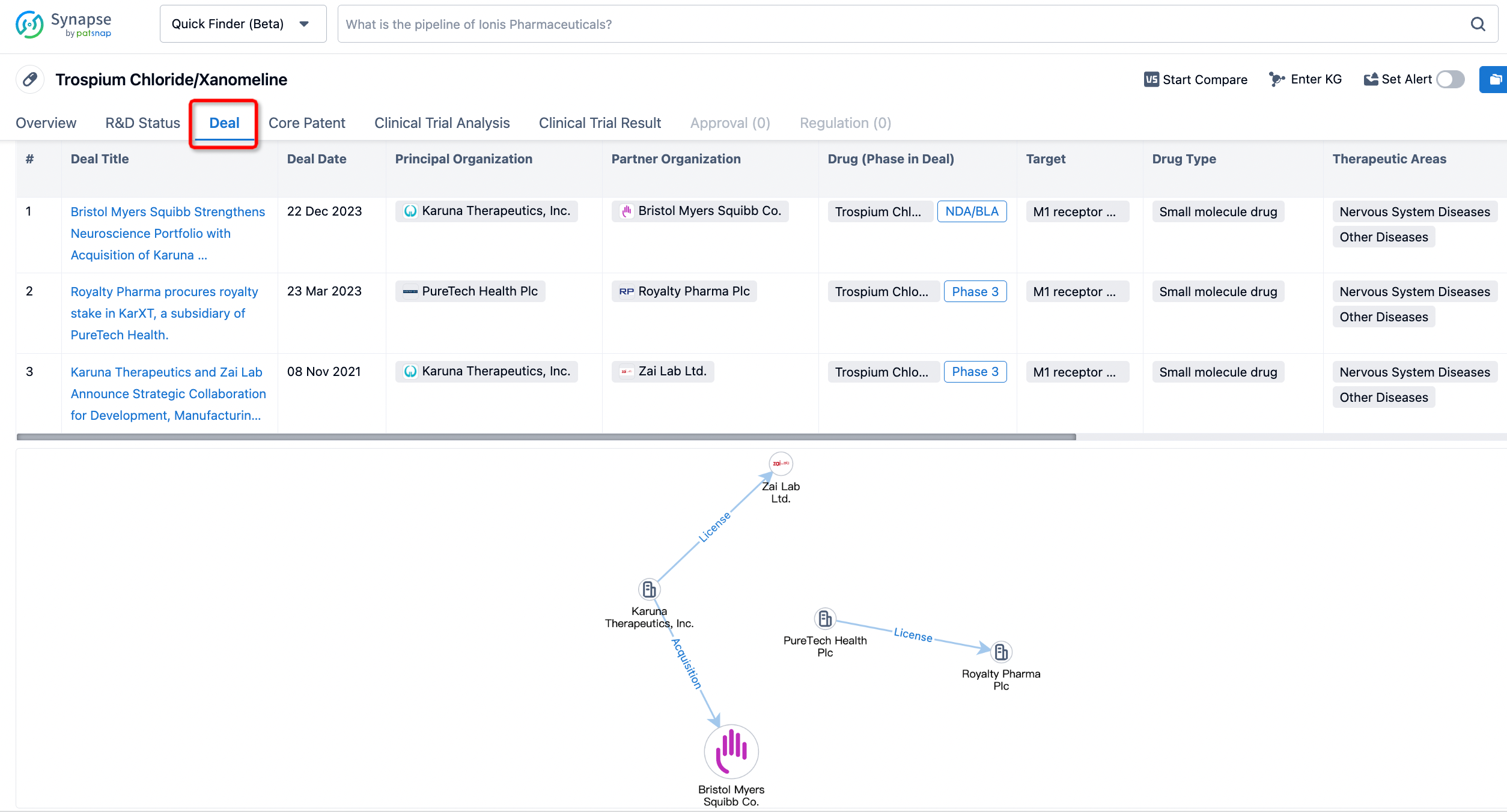
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to embark on a brand new journey of drug discovery!