Exploring TYRA-300: A Promising Orphan Drug Targeting FGFR3 in Pediatric Medicine
The small molecule drug, TYRA-300, developed by Tyra Biosciences, Inc., targets FGFR3 and falls under the category of rare pediatric disease and orphan drug regulation. The drug is being explored for various therapeutic areas including neoplasms, urogenital diseases, congenital disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, and other diseases.
As for its development stage, TYRA-300 has reached the highest phase of Phase 1/2 globally. The recognition as an orphan drug signifies its potential in addressing unmet medical needs for rare diseases or conditions. Moreover, its designation as a rare pediatric disease drug suggests its potential in treating serious or life-threatening diseases that primarily affect children, showcasing its importance in pediatric medicine.
Below, we will use the drug TYRA-300 as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
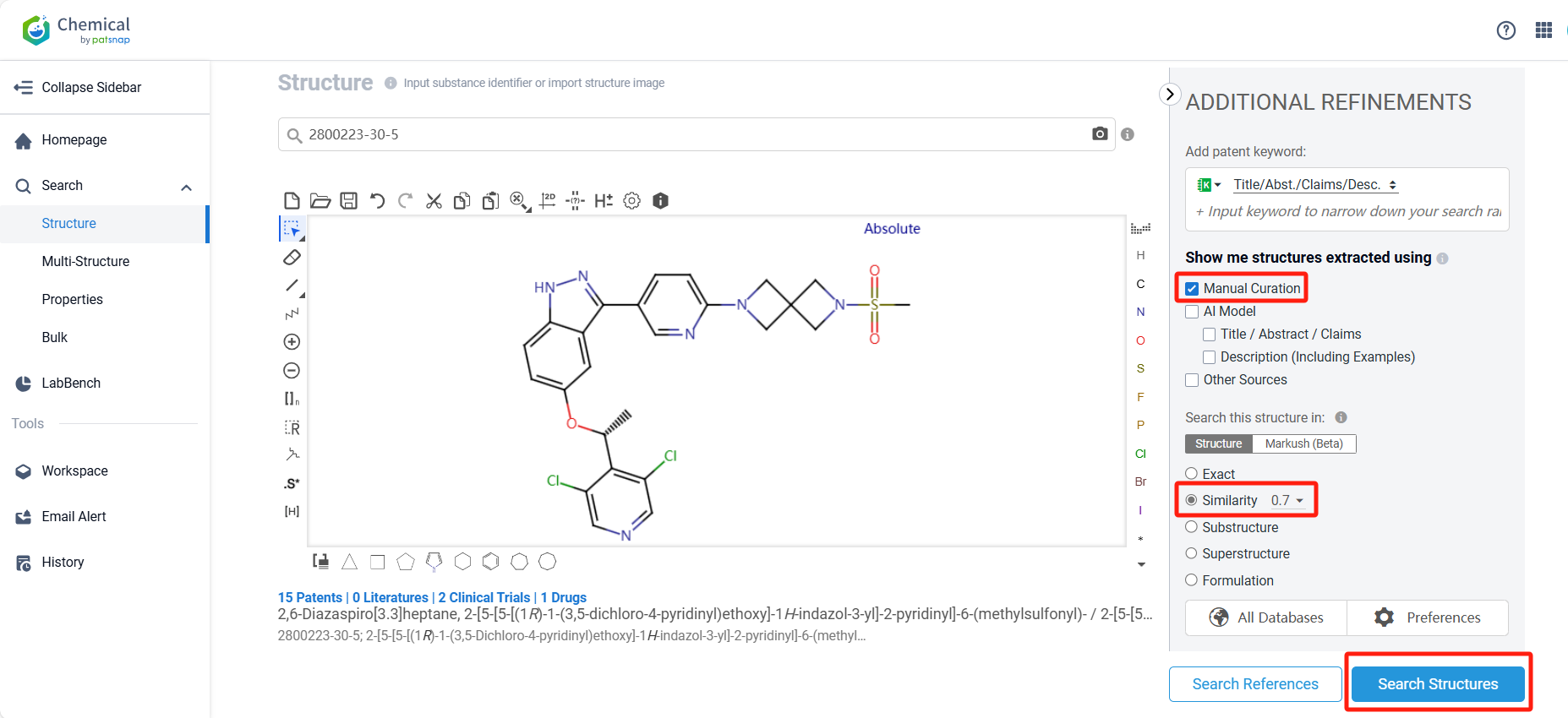
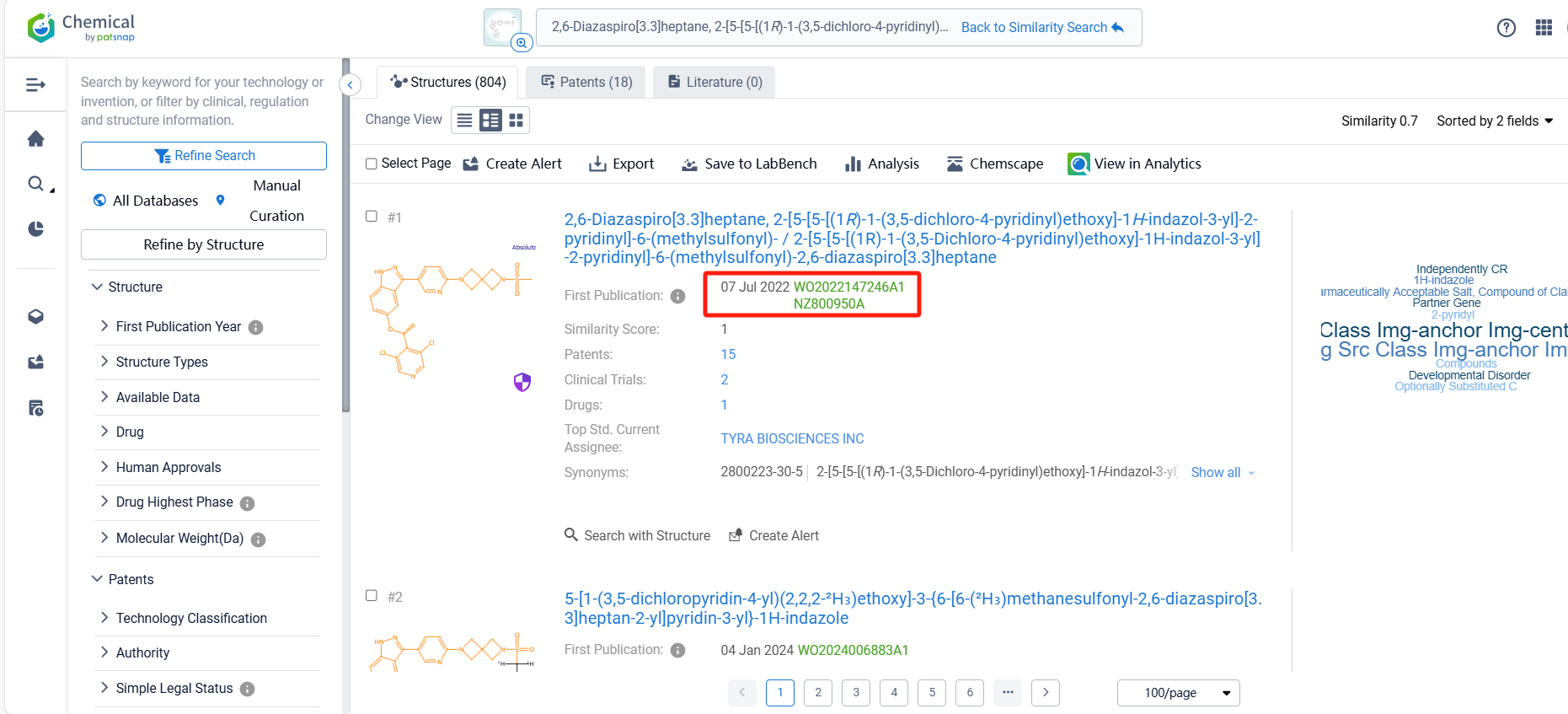
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of TYRA-300 (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, using a similarity search (setting the Tanimoto coefficient to 0.7), check the box for manual curation, click on search structures, and you can find the innovative drug TYRA-300, as disclosed in the patent application with the publication number WO2022147246A1, first made public on 2022-07-07.
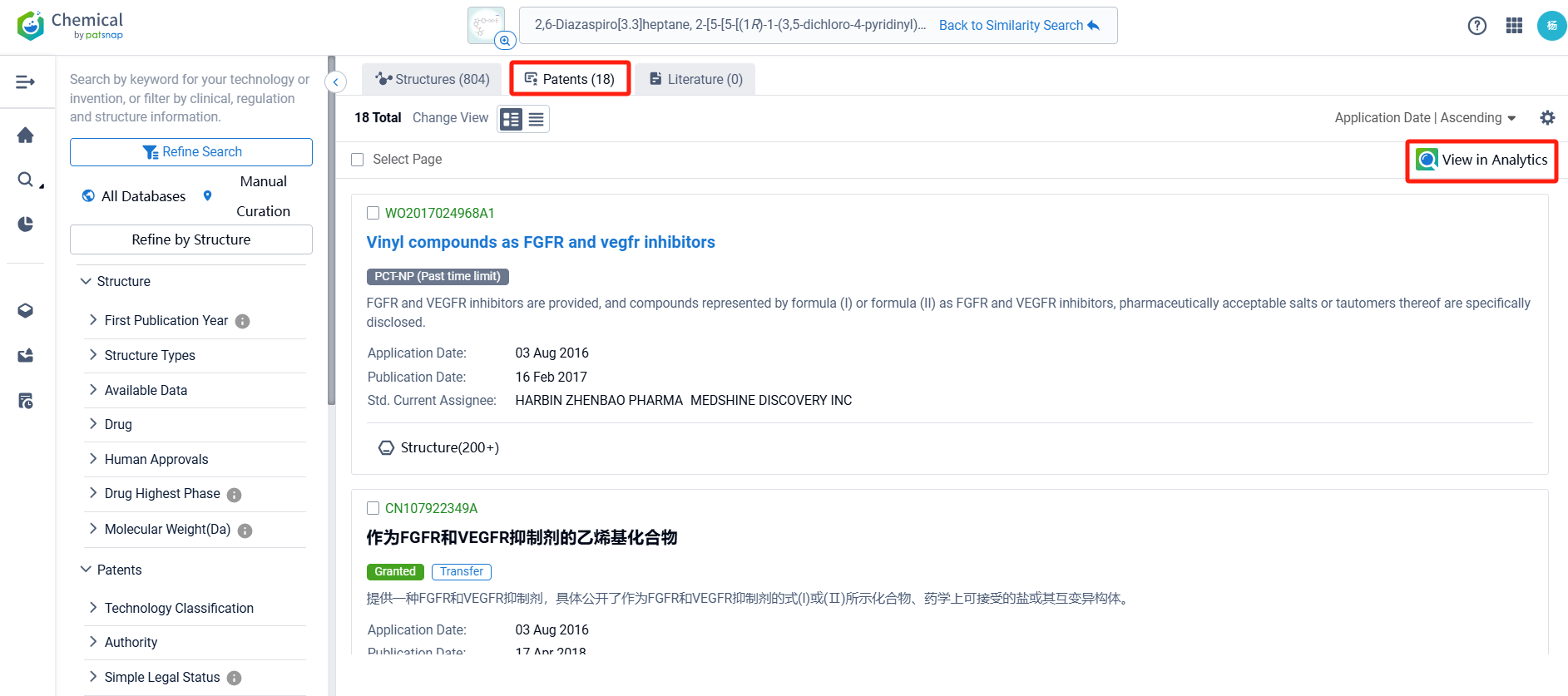
There are 18 patents related to this compound. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
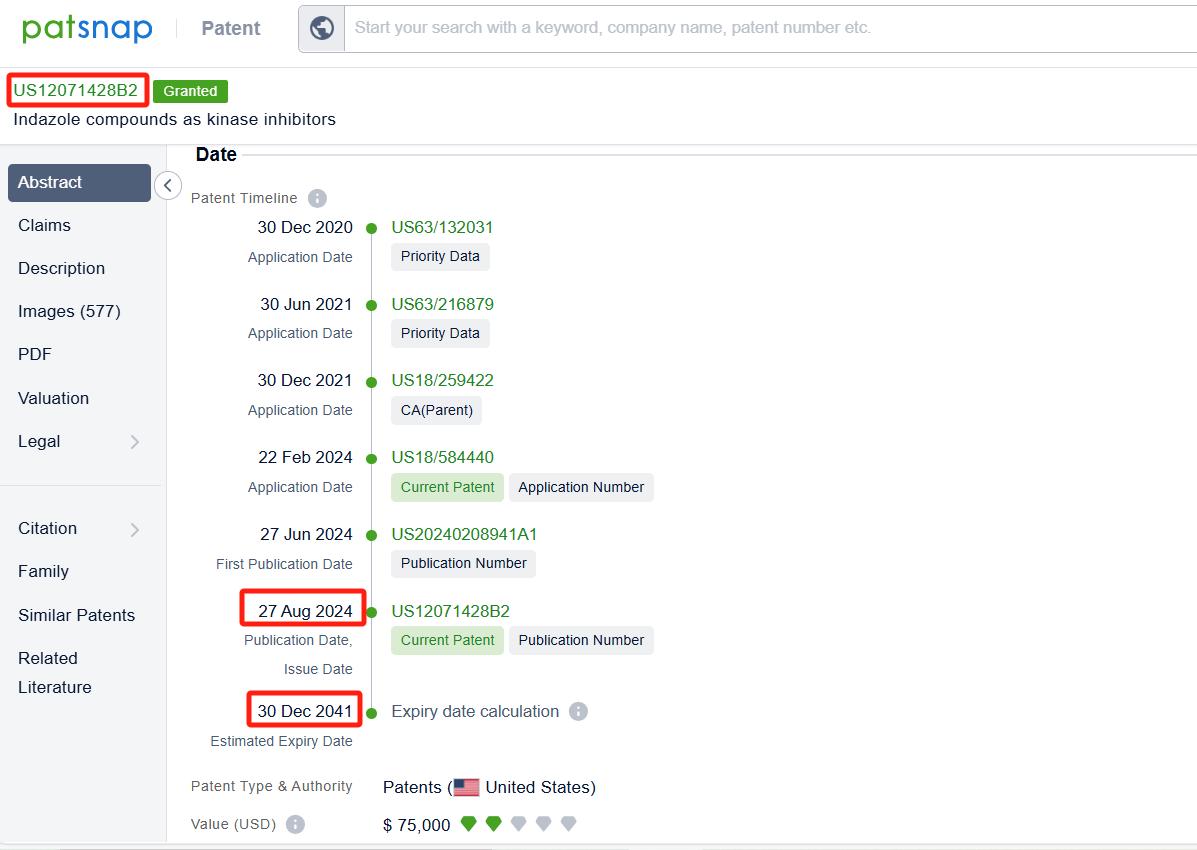
By reviewing the aforementioned patents, we can observe that the core United States patent related to this compound has been granted, with the grant publication number US12071428B2, the grant date being 27 Aug 2024, and the estimated expiration date 30 Dec 2041. The Chinese and Japanese counterparts of the compound have also entered substantive examination.
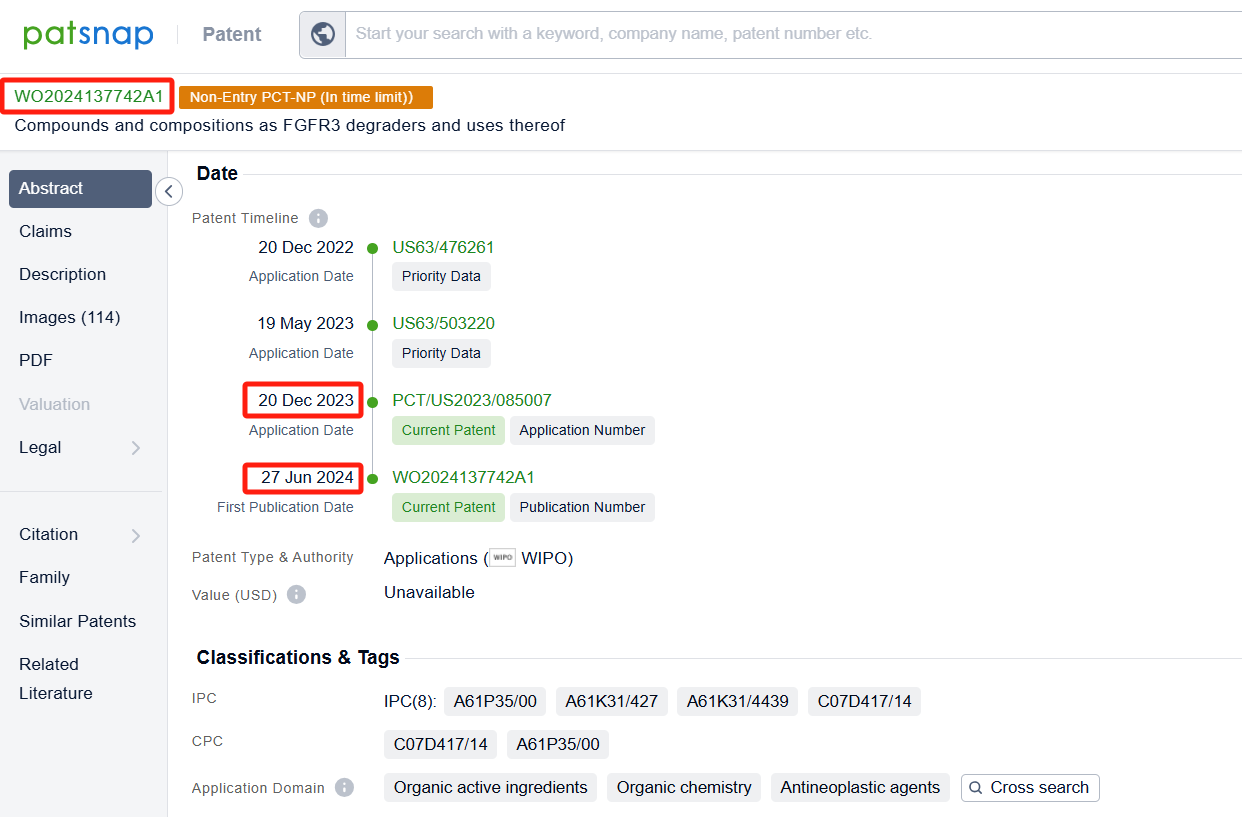
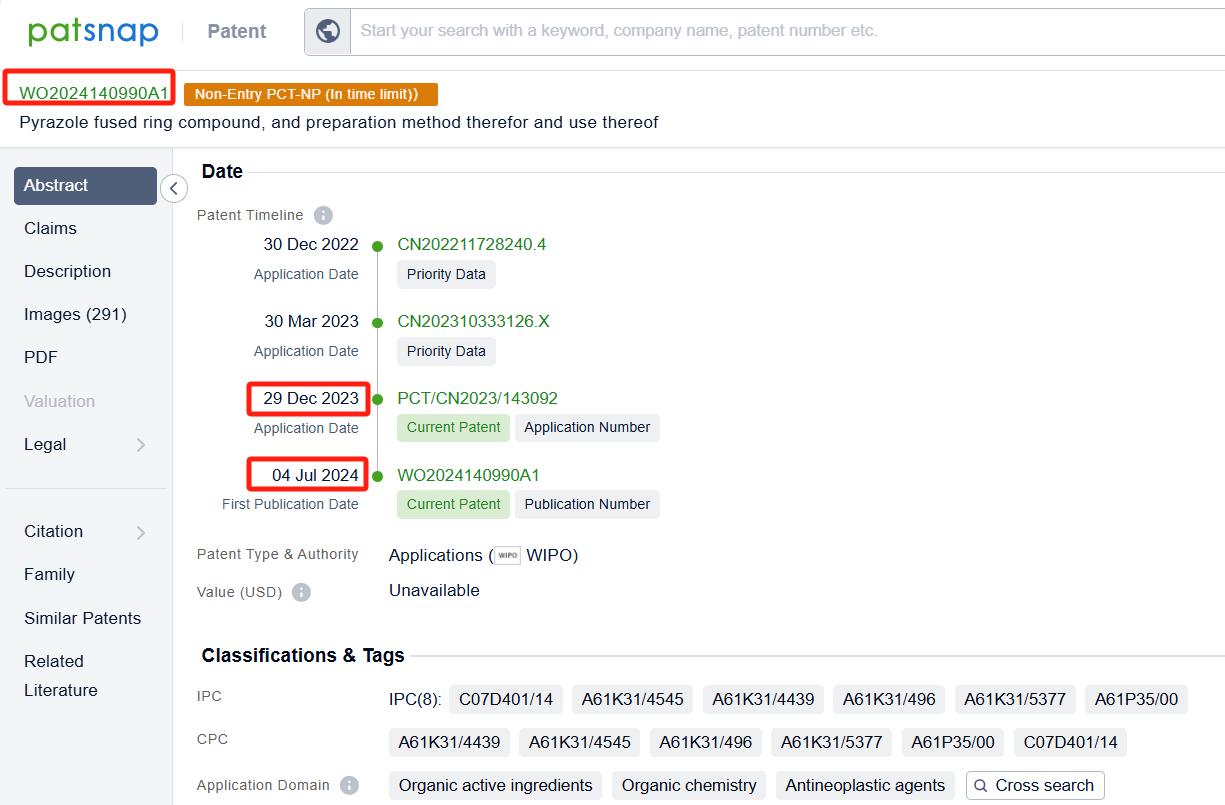
Among the applicants of the patent, one can find other companies' fast follow patents on Tyra Biosciences, Inc.; for example, Blueprint Medicines Corp.'s international patent WO2024137742A1 (application date 20231220, publication date 20240626) describes compounds and methods of using them as FGFR3 degraders for the treatment of cancer and other diseases. Additionally, Asieris Pharmaceuticals Co., Ltd.'s patent WO2024140990A1 (application date 20231229, publication date 20240704) has developed a new type of FGFR inhibitor after extensive and in-depth research. The compound of the present invention has good activity against FGFR, especially against FGFR3, and is expected to develop a new generation of FGFR inhibitors.
The drug's focus on FGFR3 as its target highlights its potential impact in addressing diseases and conditions associated with this specific biological pathway. Given the diverse therapeutic areas it aims to address, TYRA-300 possesses the potential to have a broader impact on various medical conditions, ranging from rare diseases to more prevalent cancers.
Overall, the development of TYRA-300 represents a significant advancement in the field of biomedicine, particularly in relation to the treatment of rare and complex diseases where conventional therapeutic options may be limited. Its progression to Phase 1/2 trials indicates promising prospects for its eventual approval and commercialization, offering new hope for patients affected by the diseases it aims to address.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.