Kyverna Shares Promising KYV-101 Lupus Nephritis Data at ACR 2024
Kyverna Therapeutics, Inc. (Kyverna), a biopharmaceutical firm in the clinical development phase that specializes in cell therapies for autoimmune disease patients, announced that it will share updated clinical findings from lupus nephritis (LN) patients who received KYV-101 in the ongoing Phase 1/2 trials, KYSA-1 and KYSA-3, as well as in individual patient treatments. Collaborating with prominent academic partners, Kyverna will showcase data from all six patients who were administered the target dosage of 1×10^8 CD19 CAR T cells, four of whom have undergone follow-up observations for a minimum of six months. At the six-month mark post-treatment at the target dosage, all patients continue to exhibit consistent efficacy and durability across various significant clinical endpoints.
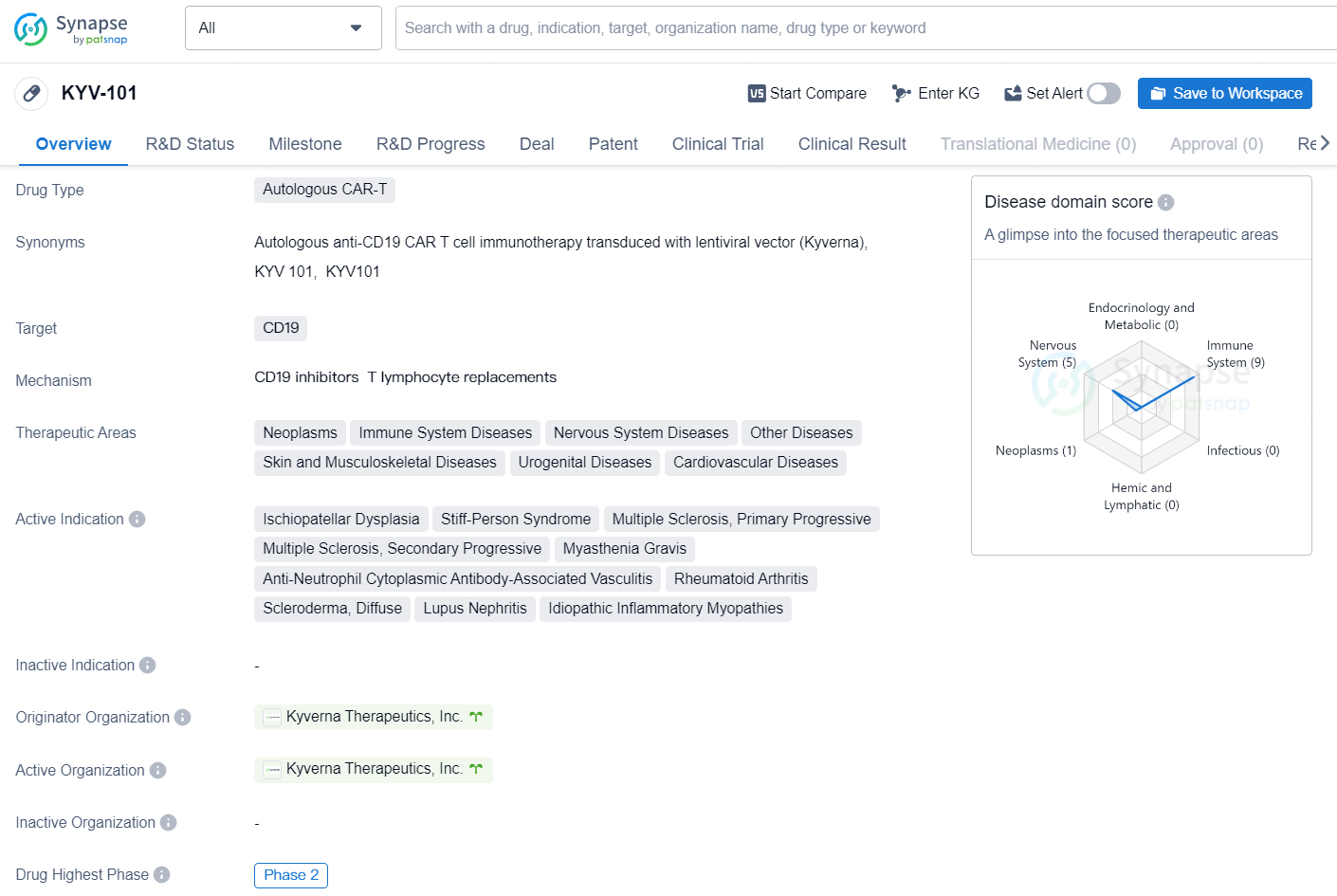
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The forthcoming updates will be showcased at a corporate symposium named, “KYV-101 Anti-CD19 CAR T-Cell Therapy: The Future of Autoimmune Disease Treatment,” set to occur at 5:45 PM ET on November 18, 2024. Following the symposium, slides from the presentation will be made available on the company's website.
"Patients with lupus nephritis endure a significant disease burden linked to high rates of morbidity and mortality, with as many as 30% facing end-stage renal disease that necessitates dialysis or kidney transplantation," remarked Prof. Georg Schett, M.D. from Friedrich-Alexander-University in Erlangen, Germany, who is one of the speakers. "The findings support that treatment with KYV-101 achieves substantial B cell depletion in patients with LN, seems to reset the immune system, stabilizes eGFR, maintains kidney functionality, and facilitates clinical improvement in SLE manifestations. Importantly, these clinical advantages arise while also reducing immunosuppressants and tapering glucocorticoids to physiological levels, all within a manageable safety framework."
"As we accumulate more data from our KYV-101 clinical trials, we are becoming increasingly adept at identifying the right patients to receive the appropriate dosage and protocol. We are eager to present these new findings, which further validate KYV-101’s potential to provide lasting and transformative results for lupus nephritis patients, including those facing severe and chronic forms of the disease,” stated Warner Biddle, Chief Executive Officer of Kyverna.
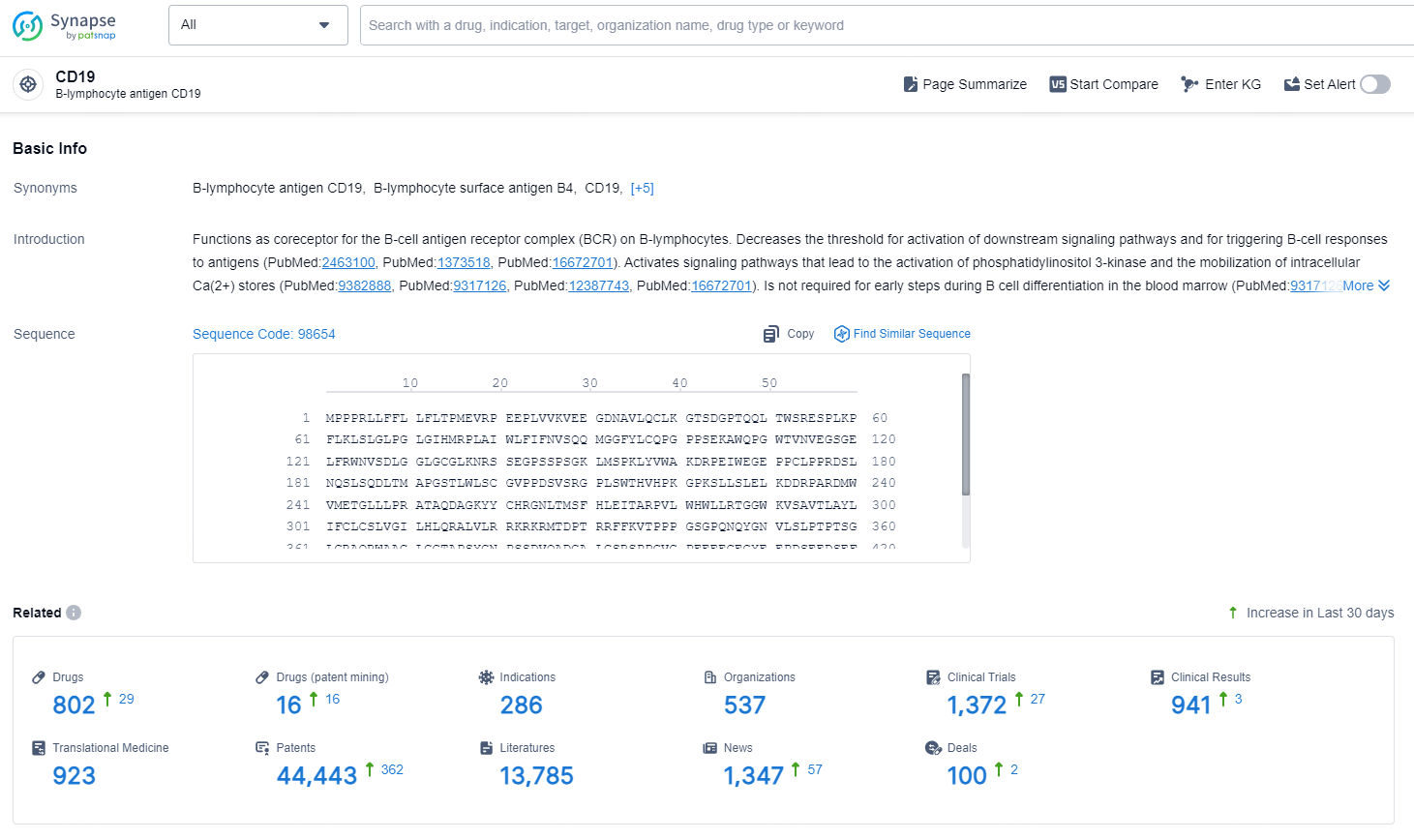
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of November 18, 2024, there are 802 investigational drugs for the CD19 target, including 286 indications, 537 R&D institutions involved, with related clinical trials reaching 1372, and as many as 44443 patents.
KYV-101 is an investigational chimeric antigen receptor T-cell (CAR T) therapy being developed by Kyverna Therapeutics for treating autoimmune diseases, particularly lupus nephritis (LN). LN is a severe kidney complication of systemic lupus erythematosus (lupus), affecting about 40% of adults diagnosed with the condition. It often proves refractory to standard treatments, leading to kidney failure in some cases.