Spirovant Launches Phase 1/2 Trial of Aerosol Genetic Therapy for Cystic Fibrosis
Spirovant Sciences, a company focused on gene therapy in clinical development for genetic respiratory disorders, has announced that the initial patient has received treatment in its SAAVe Phase 1/2 clinical trial of SP-101 combined with Augmenter for cystic fibrosis therapy.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
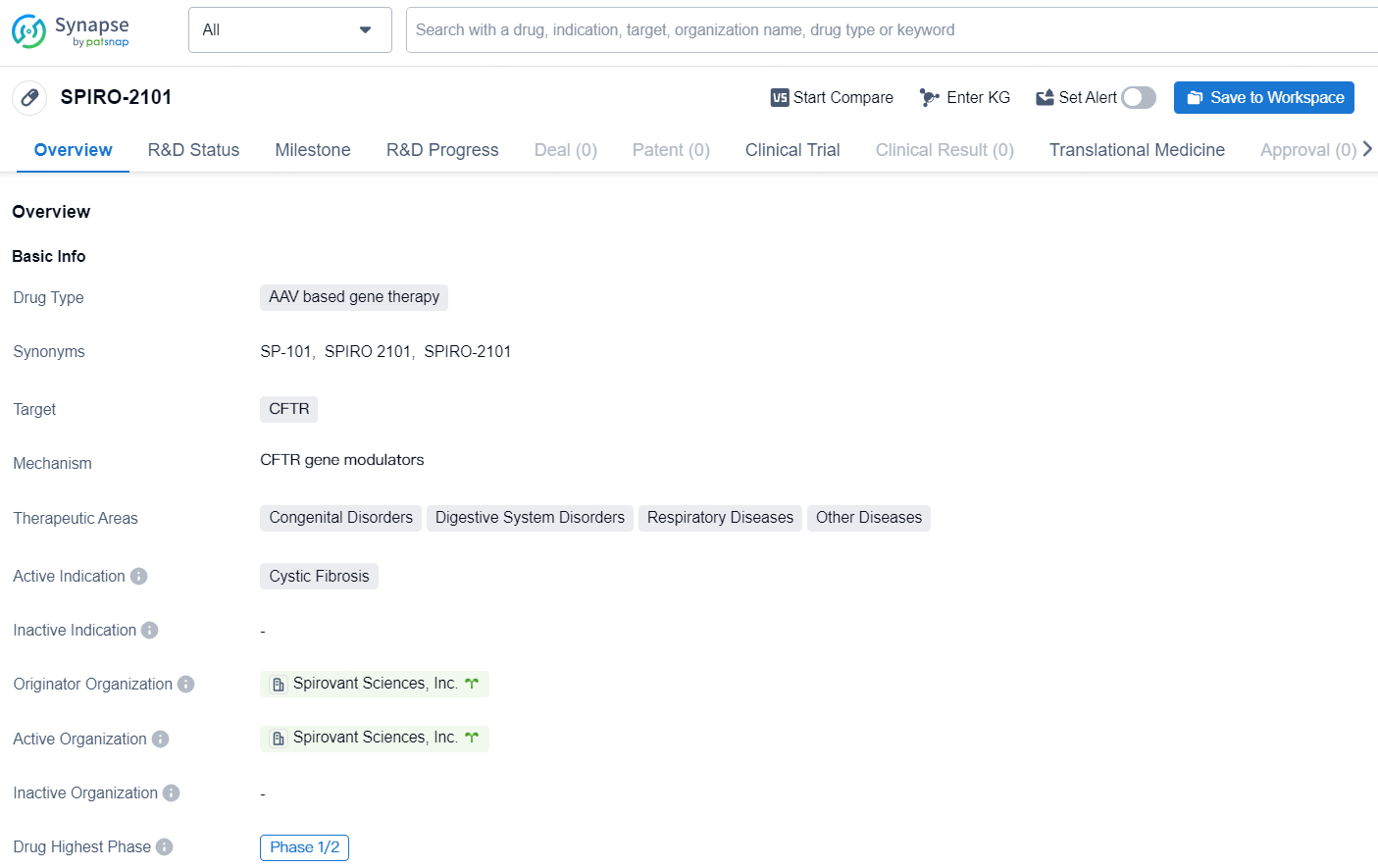
SP-101, an investigational gene therapy delivered through inhalation, utilizes a novel recombinant adeno-associated virus (AAV) designed for effective targeting of human airway epithelia. The therapy employs doxorubicin as an augmenter to significantly enhance the expression of the functional cystic fibrosis transmembrane conductance regulator (CFTR) transgene, aiming for levels that could yield substantial clinical benefits.
"This marks a significant achievement for Spirovant and the cystic fibrosis community with the administration of the first patient in the SAAVe Phase 1/2 clinical trial," stated Roland Kolbeck, PhD, Chief Scientific Officer at Spirovant Sciences. "This treatment approach is mutation-agnostic, presenting an opportunity to address the needs of a diverse group of cystic fibrosis patients, including those who cannot utilize CFTR modulators and those who do not experience adequate results from them."
About 10% of cystic fibrosis patients currently do not find relief with available CFTR modulator therapies, leaving them without effective treatment alternatives. SP-101 has been specifically refined to tackle previously encountered obstacles to gene therapy in cystic fibrosis, with the goal of treating the core issues associated with cystic fibrosis lung disease, thus providing benefits to a wide array of patients, regardless of their specific CFTR mutations.
"Dosing the first patient with this innovative gene therapy candidate marks an important forward movement for the cystic fibrosis community we aim to support," said Claire Keating, MD, an Associate Professor of Medicine and Co-Director of the Gunnar Esiason Adult Cystic Fibrosis and Lung Center at Columbia University Irving Medical Center. “This successful initial dosing is a pivotal milestone, paving a new potential therapeutic avenue that brings us closer to our aim of effectively treating individuals with CF, particularly those who are currently not candidates for existing highly effective therapies. I extend my sincere gratitude to our patient and all participants contributing to this research, for it is their involvement that is crucial for the advancement of new CF treatments," Dr. Keating added.
The SAAVe Phase 1/2 clinical trial is a multicenter, open-label study focusing on escalating and expanding dosages of SP-101 + Augmenter in cystic fibrosis patients who are ineligible for CFTR modulator therapy or insufficiently benefitting from it. The primary endpoints of the trial include safety and tolerability, with secondary endpoints assessing lung function (ppFEV1), quality of life (CFQ-R), and bronchoscopy biomarkers.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
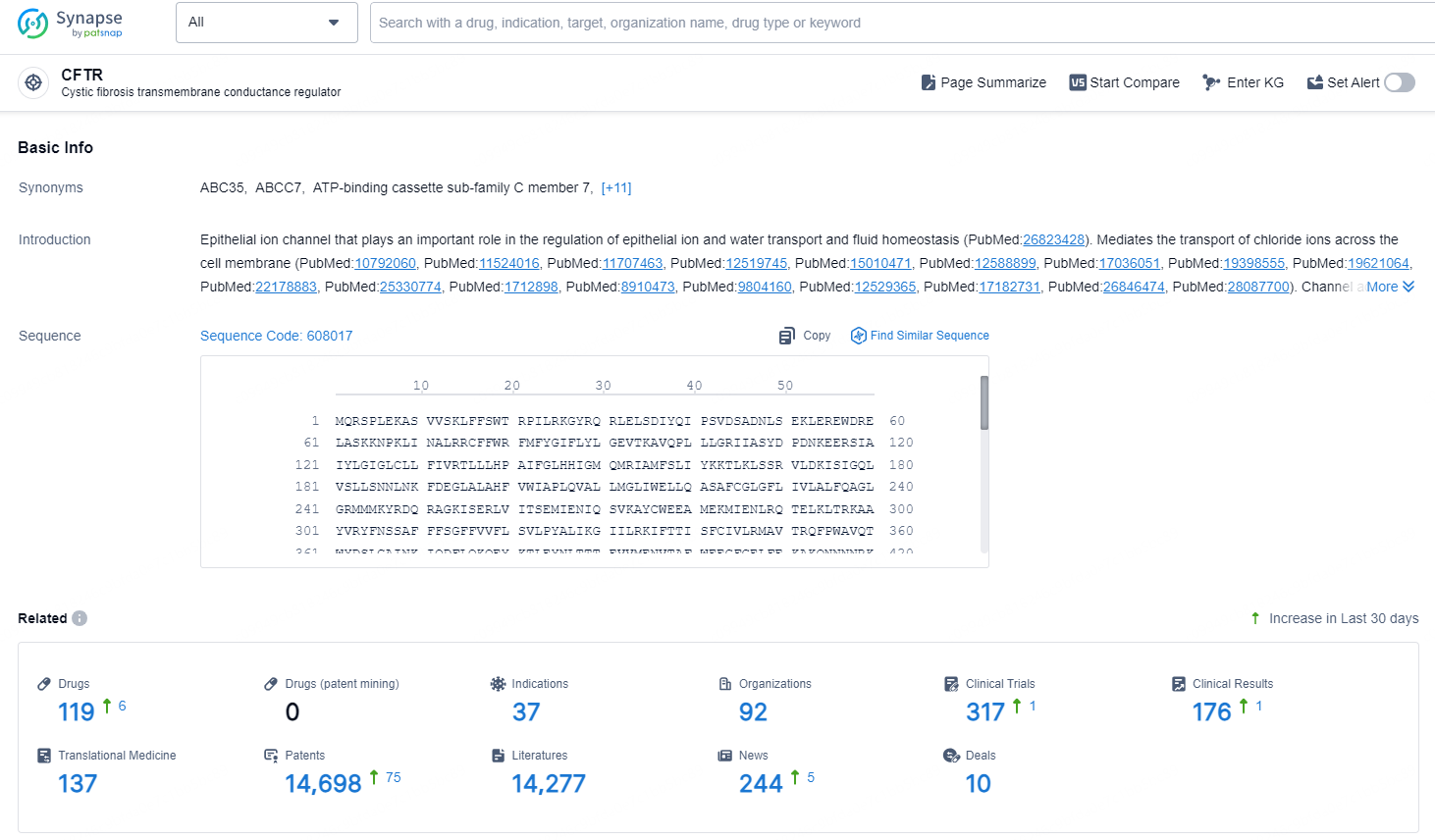
According to the data provided by the Synapse Database, As of November 18, 2024, there are 119 investigational drugs for the CFTR target, including 37 indications, 92 R&D institutions involved, with related clinical trials reaching 317, and as many as 14698 patents.
The drug SPIRO-2101 is an AAV based gene therapy that targets the CFTR gene. It is designed to treat patients with Cystic Fibrosis, and its therapeutic areas also include Congenital Disorders, Digestive System Disorders, Respiratory Diseases, and Other Diseases. The originator organization of SPIRO-2101 is Spirovant Sciences, Inc., and it is currently in the highest phase of clinical development at Phase 1/2 globally.