Exploring Vincamine's Revolutionary R&D Successes
Vincamine's R&D Progress
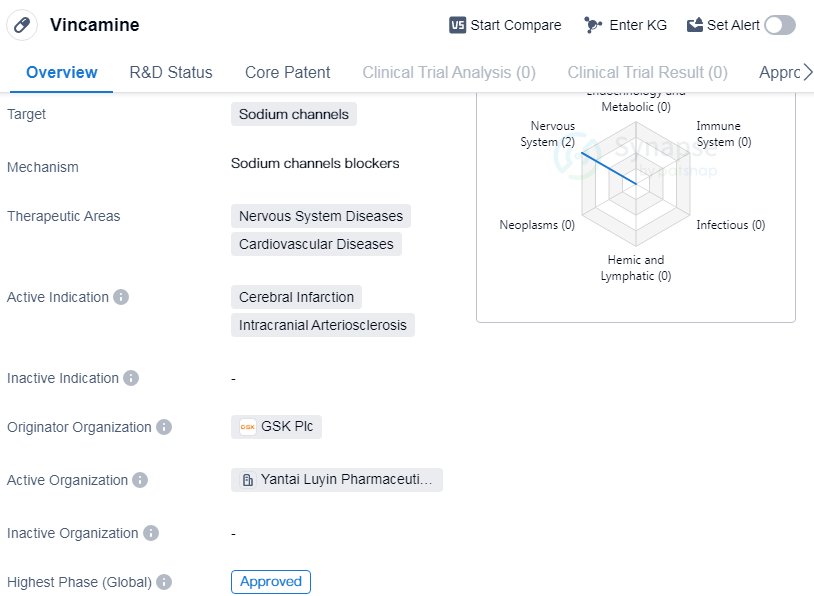
Vincamine is a small molecule drug that targets sodium channels and is primarily used in the treatment of nervous system diseases and cardiovascular diseases. It is specifically indicated for cerebral infarction and intracranial arteriosclerosis. The drug was first approved in China in January 2000 and has since received approval in other countries as well.
Vincamine is developed by GSK Plc, a renowned pharmaceutical company. It is classified as a small molecule drug, which means it consists of low molecular weight compounds that can easily penetrate cell membranes. This characteristic allows for efficient drug delivery and interaction with the target sodium channels.
Vincamine's primary therapeutic areas are nervous system diseases and cardiovascular diseases. Nervous system diseases encompass a wide range of conditions affecting the brain, spinal cord, and nerves, while cardiovascular diseases refer to disorders of the heart and blood vessels. Vincamine's specific indications are cerebral infarction and intracranial arteriosclerosis,which are both related to the nervous system and cardiovascular system.
Cerebral infarction is a type of stroke caused by a blockage in the blood vessels supplying the brain, leading to a lack of oxygen and nutrients. Intracranial arteriosclerosis, on the other hand, refers to the hardening and narrowing of the arteries within the skull, which can impede blood flow to the brain.
Vincamine has successfully completed the highest phase of clinical trials and has been approved for use globally. The drug's approval in China in 2000 indicates its early adoption and recognition in the country.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Vincamine: Sodium channel blockers
Sodium channel blockers are a class of drugs that inhibit the activity of sodium channels in the body. Sodium channels are responsible for the movement of sodium ions across the cell membrane, playing a crucial role in the generation and propagation of electrical signals in nerve cells and cardiac cells.
From a biomedical perspective, sodium channel blockers are commonly used in the field of cardiology to treat various heart conditions, such as arrhythmias (abnormal heart rhythms) and angina (chest pain). By blocking sodium channels, these medications help to slow down the electrical impulses in the heart, thereby regulating the heart rate and reducing the risk of abnormal rhythms.
In addition to their cardiovascular applications, sodium channel blockers can also be used as local anesthetics. By blocking sodium channels in sensory neurons, these drugs prevent the transmission of pain signals, leading to temporary numbness in the area where they are applied.
It's important to note that sodium channel blockers can have side effects, such as dizziness, nausea, and changes in heart rhythm. Therefore, their use should be carefully monitored and prescribed by healthcare professionals.
Drug Target R&D Trends for Vincamine
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 125 Sodium channels drugs worldwide, from 155 organizations, covering 92 indications, and conducting 1019 clinical trials.
The analysis of the current competitive landscape and future development of target Sodium channels reveals that multiple companies are growing rapidly under this target. The indications for these drugs cover a wide range of therapeutic areas, suggesting a diverse market for Sodium channel-targeting drugs.
Small molecule drugs are progressing most rapidly under the current targets, indicating intense competition in the development of innovative drugs. The presence of biosimilars is not significant, further emphasizing the focus on small molecule drugs.
China has shown significant progress in the development of drugs targeting sodium channels, with the highest number of approved drugs. The United States and Japan also have a considerable presence in this field. Other countries such as the European Union, the United Kingdom, and France are also actively developing drugs under this target.
Overall, the analysis suggests a competitive landscape in the development of Sodium channel-targeting drugs, with multiple companies and countries actively involved.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Vincamine is a small molecule drug developed by GSK Plc that targets sodium channels. It is primarily used in the treatment of nervous system diseases and cardiovascular diseases, specifically cerebral infarction and intracranial arteriosclerosis. The drug has received global approval, and was first approved in 2000.