Fam-trastuzumab deruxtecan-NXKI: brief review of its R&D progress and the clinical result in 2023 ESMO
The disclosure of the clinical trial of Trastuzumab deruxtecan (T-DXd) for the pretreatment of patients (pts) with her2-expressing solid tumors at ESMO 2023 provides the factual basis for subsequent, more in-depth studies.
Fam-trastuzumab deruxtecan-NXKI's R&D Progress
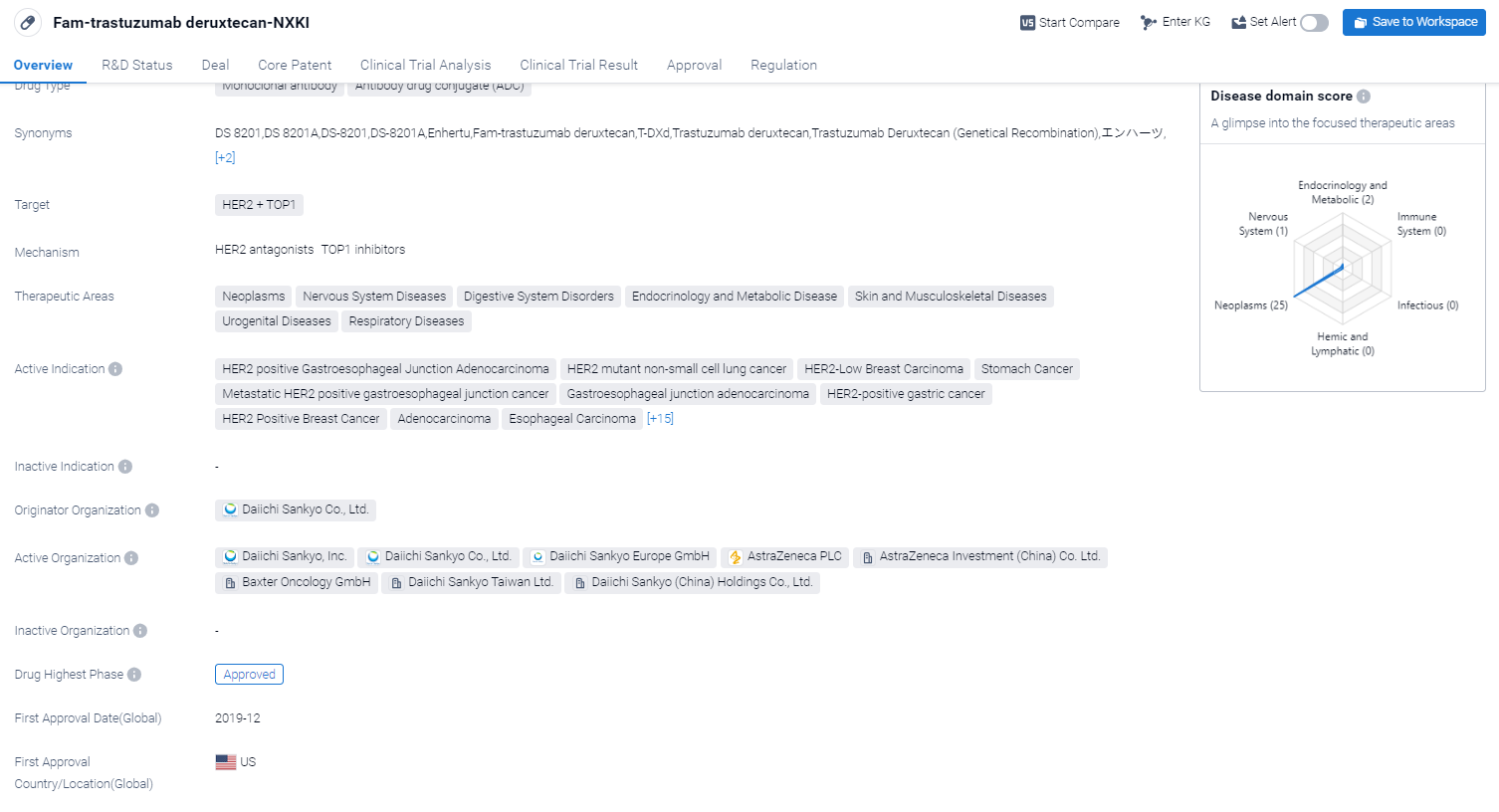
Fam-trastuzumab deruxtecan-NXKI is a drug classified as a monoclonal antibody and an antibody drug conjugate (ADC). It targets HER2 and TOP1, making it suitable for the treatment of various diseases. The drug has shown potential in therapeutic areas such as neoplasms, nervous system diseases, digestive system disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, urogenital diseases, and respiratory diseases.
According to the Patsnap Synapse, Fam-trastuzumab deruxtecan-NXKI was developed by Daiichi Sankyo Co., Ltd., and it has received approval for use in the highest phase in both global and Chinese markets. And the clinical trial areas for Fam-trastuzumab deruxtecan-NXKI are primarily in the United States, China, and United Kingdom. The key indication is Neoplasms.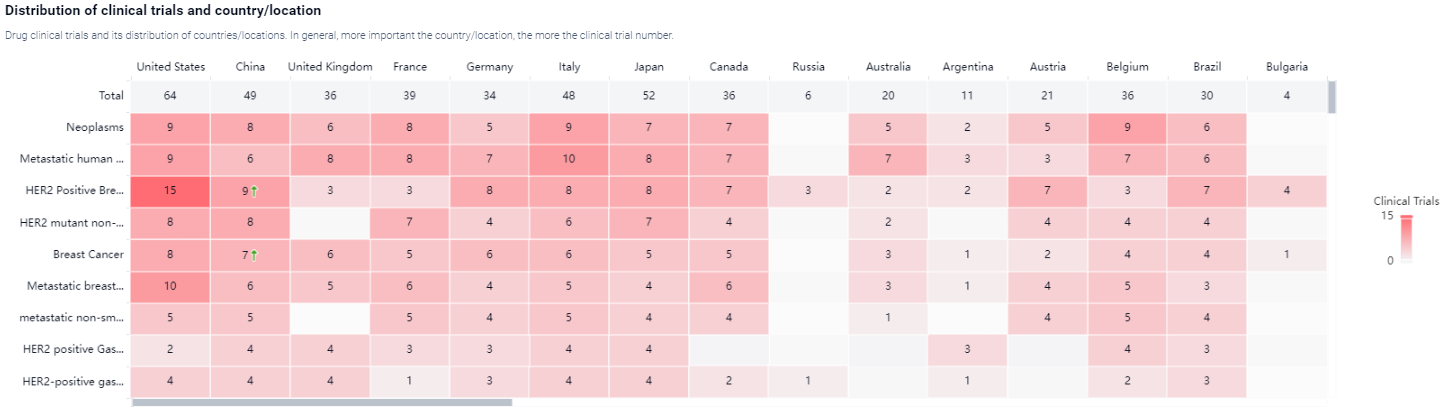
Detailed Clinical Result of Fam-trastuzumab deruxtecan-NXKI
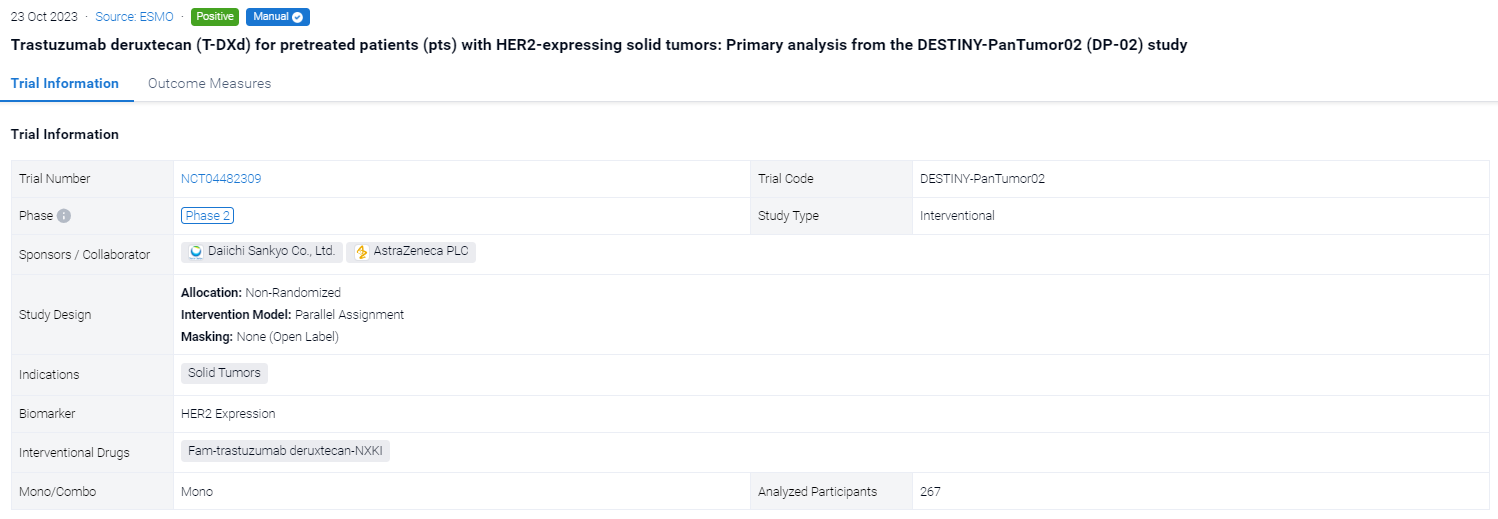
This open-label, Phase 2 study (NCT04482309) evaluated T-DXd (5.4 mg/kg Q3W) in pts with HER2-expressing (immunohistochemistry [IHC] 3+/2+ by local or central testing), locally advanced/metastatic disease after ≥1 systemic treatment (Tx), or without alternative Tx options.
In this study, primary endpoint was investigator-assessed confirmed ORR. Secondary endpoints included safety, DOR, PFS and OS.
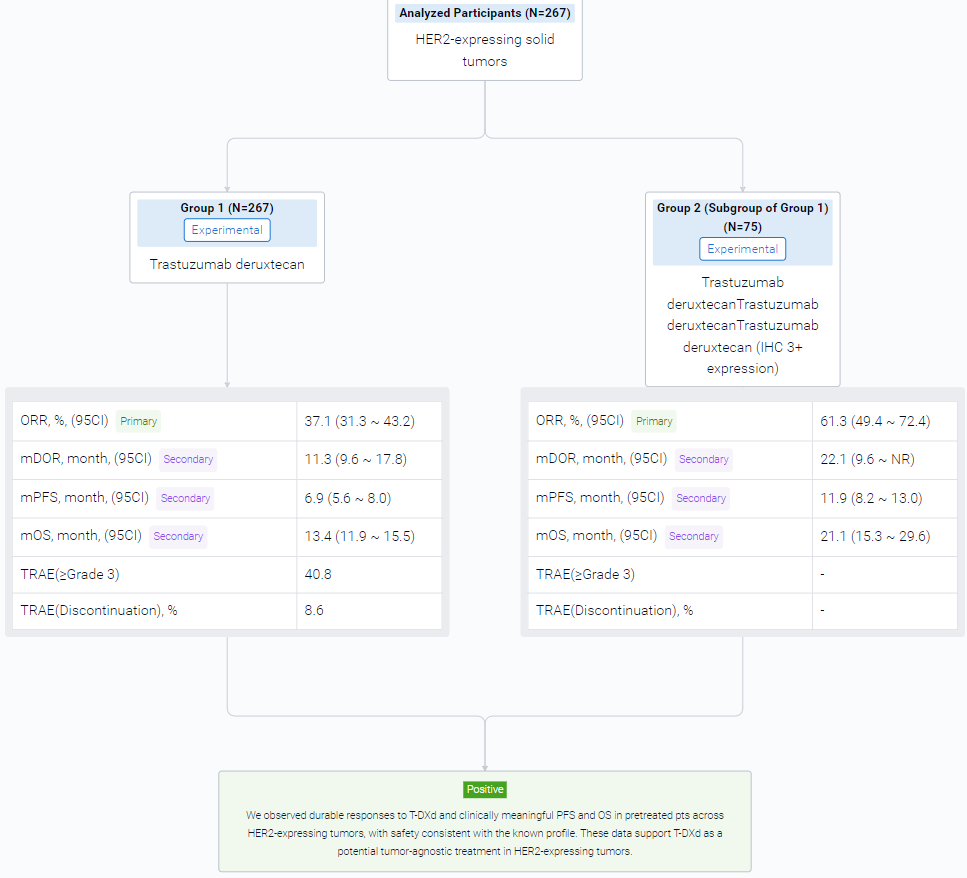
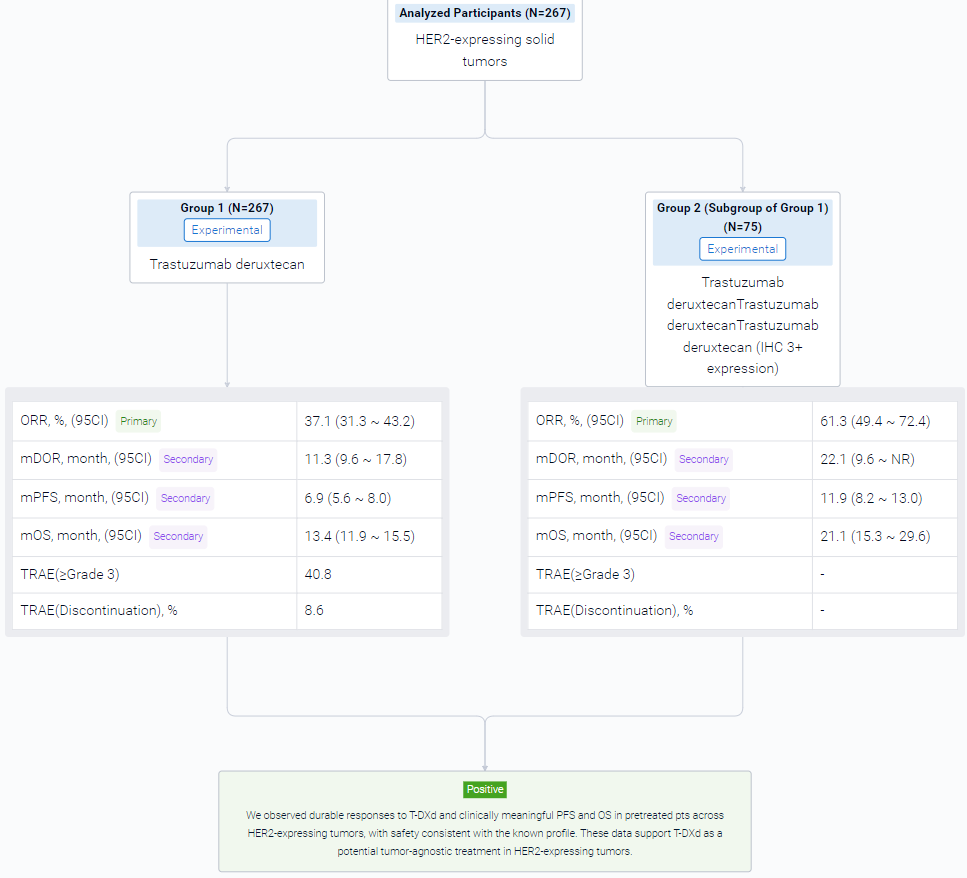
The result showed that at data cut off (Jun 2023), 267 pts with biliary tract (BTC), bladder (URO), cervical (CC), endometrial (EC), ovarian (OC), pancreatic (PC), or other tumors had received Tx (median [m] follow up: 12.75 [range 0.4–31.6] months [mo]); 72.3% received ≥2 prior lines of therapy. In all pts, investigator-assessed ORR (95% CI) was 37.1% (31.3, 43.2); mDOR (95% CI) was 11.3 mo (9.6, 17.8); mPFS (95% CI) was 6.9 mo (5.6, 8.0); and mOS (95% CI) was 13.4 mo (11.9, 15.5). In pts with IHC 3+ expression (central; n=75) ORR was 61.3% (49.4, 72.4); mDOR was 22.1 mo (9.6, not reached); mPFS was 11.9 mo (8.2, 13.0); and mOS was 21.1 mo (15.3, 29.6). Table shows ORR, PFS and OS by tumor type in all pts and IHC 3+. Grade (G) ≥3 Tx-related adverse events (AEs) occurred in 40.8% of pts; 8.6% discontinued Tx due to Tx-related AEs. Adjudicated Tx-related interstitial lung disease/pneumonitis occurred in 10.5% (n=28) of pts (9.0% [n=24] G≤2; 1.1% [n=3] G5).
It can be concluded that T-DXd has a durable effect and clinically meaningful PFS and OS in pretreated pts across HER2-expressing tumors, with safety consistent with the known profile. These data support T-DXd as a potential tumor-agnostic treatment in HER2-expressing tumors.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
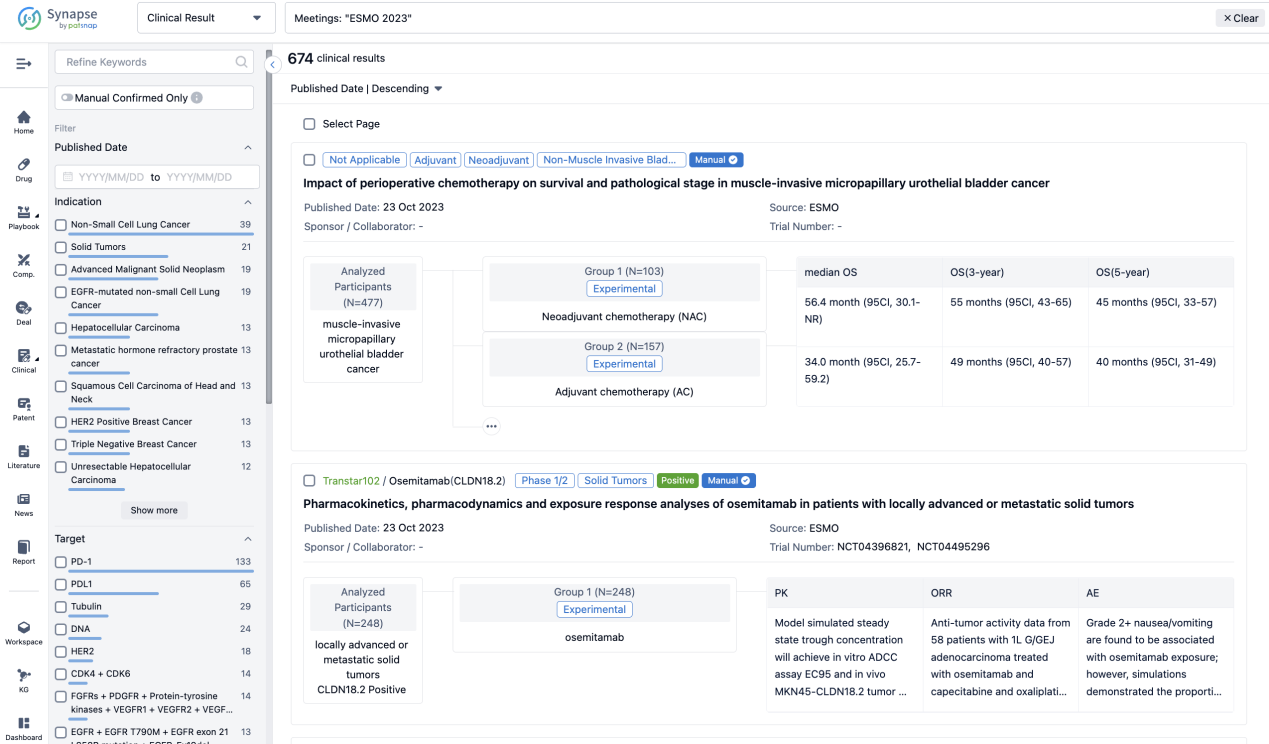
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
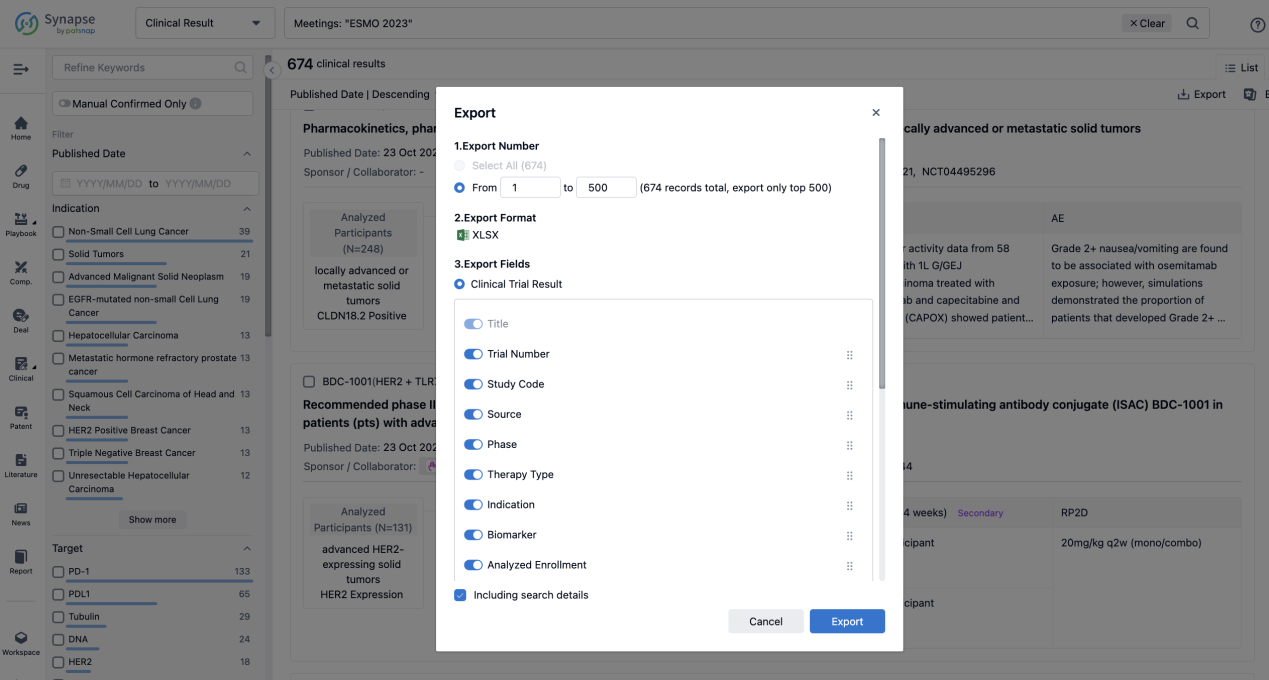
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!