The US FDA has approved Takeda's ADZYNMA for treating Congenital Thrombotic Thrombocytopenic Purpura (cTTP)
Takeda has revealed that the FDA has given its approval for ADZYNMA (ADAMTS13, recombinant-krhn) to be utilized for preventive and necessary treatments for grown-ups and children suffering from congenital thrombotic thrombocytopenic purpura. Standing as the debut and sole FDA-authorized recombinant ADAMTS13 protein, ADZYNMA aims to fulfill a medical necessity in those affected by cTTP by compensating for the lacking ADAMTS13 enzyme.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Individuals impacted by cTTP have been facing high-risk, fatal health predicaments without any authorized specialized remedies until now. This has been a concerning matter," Julie Kim, head of the U.S. Business Division and U.S. country leader at Takeda, articulated.
Kim went on to say, "Our mission is to assist patients who don't have many or any pharmaceutical alternatives, and it’s an empowering challenge to craft cutting-edge solutions for rare illnesses. This is a path Takeda, as a hematology pioneer, has been following for over seven decades. We are now elated to increase our commitment to the rare disease populace by introducing ADZYNMA, the first FDA-sanctioned medicinal choice for those battling cTTP."
Spero R. Cataland, M.D., an internal medicine professor at Wexner Medical Center at The Ohio State University, weighed in, stating, "Over the past several decades, substantial strides have been made in comprehending the association between ADAMTS13 shortage and cTTP. This has finally led us to a period where we have an FDA-validated treatment option for individuals living with this uncommon condition.”
"ADZYNMA gives patients a supplement for their deficient ADAMTS13 enzyme while offering a commendable effectiveness and safety pattern. It also necessitates less time and volume for administration in contrast to the existing plasma-powered therapies. This day signifies a great accomplishment, opening up new avenues for the cTTP patient community," added Cataland.
FDA's endorsement of ADZYNMA was a result of holistic evidence based on the review of Phase 3 trial's data on efficacy, pharmacokinetics, safety, and tolerability, and the follow-up study of cTTP.
In the stage 3 clinical trial, patients were given a 40 IU/kg dosage of ADZYNMA IV or a plasma-based therapy biweekly or weekly based on the protocol they were initially registered under for the first six months. They then proceeded to an alternative therapy for the next six months, and all patients were subjected to ADZYNMA for the concluding six months.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
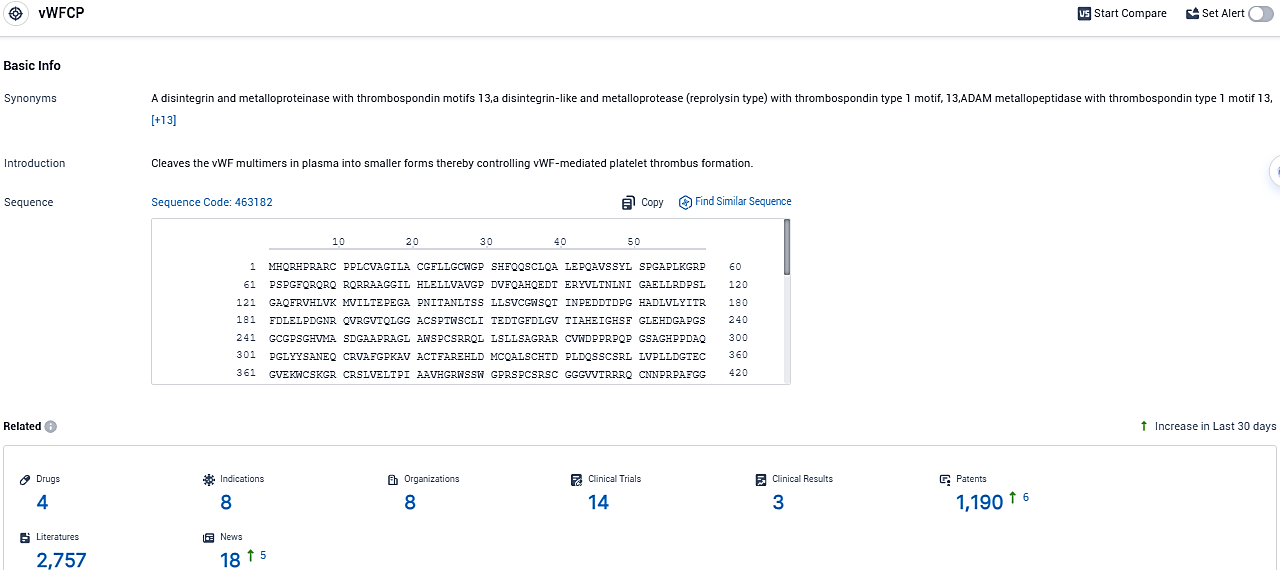
According to the data provided by the Synapse Database, As of November 18, 2023, there are 4 investigational drugs for the vWFCP target, including 8 indications, 8 R&D institutions involved, with related clinical trials reaching 14, and as many as 1190 patents.
ADZYNMA was previously granted Orphan Drug Designation by the U.S. FDA for the treatment and prevention of TTP, including its acquired idiopathic and secondary forms, as well as Fast Track and Rare Pediatric Disease Designation. The U.S. FDA granted Takeda a Rare Pediatric Disease Voucher for the approval of ADZYNMA. ADZYNMA has also been granted ODD by EMA and Japan’s Ministry of Health, Labour and Welfare for the treatment of TTP.