Favorable Phase 1 Results for Gan & Lee's GZR18 Tablet: 4.16% Weight Loss in Two Weeks
Gan & Lee Pharmaceuticals reported favorable preliminary outcomes from a Phase 1 clinical trial of its oral glucagon-like peptide-1 receptor agonist (GLP-1 RA) GZR18 tablets involving healthy subjects in China.
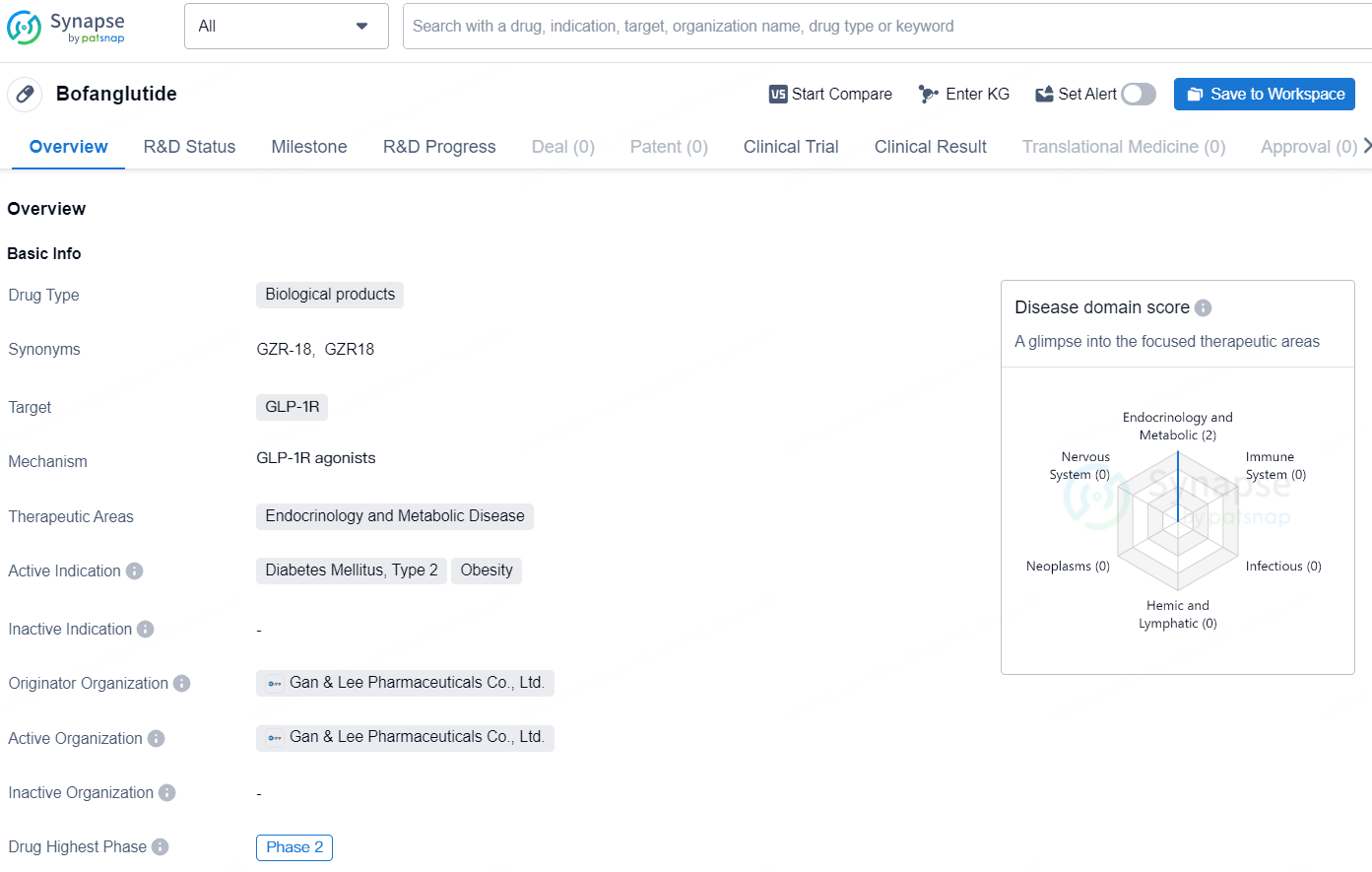
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The Phase 1 clinical trial (CTR20240663) was a randomized, open-label study involving 92 healthy adults, aimed at evaluating the bioavailability, pharmacokinetics (PK), pharmacodynamics (PD), safety, and tolerability of GZR18 tablets. The research also investigated how meal timing influences the drug's PK, PD, safety, and tolerability. Results demonstrated that GZR18 tablets were both safe and well-tolerated, with gastrointestinal issues being the most frequently reported adverse events, aligning with the known safety profile of GLP-1 based treatments.
The pharmacokinetic data indicated good absorption, which supports the feasibility of a once-daily oral dosage. Both single and multiple doses exhibited a dose-response correlation concerning PK and PD metrics. Individuals receiving the targeted daily dose of 60 mg for a fortnight showed an average weight loss of 4.16% from their initial measurements. Moreover, one week after ceasing treatment, participants experienced an additional weight reduction of 0.51%, culminating in an average overall loss of 4.67% compared to their baseline. Even 21 days post-treatment, neither the participants' body weight nor BMI returned to baseline levels.
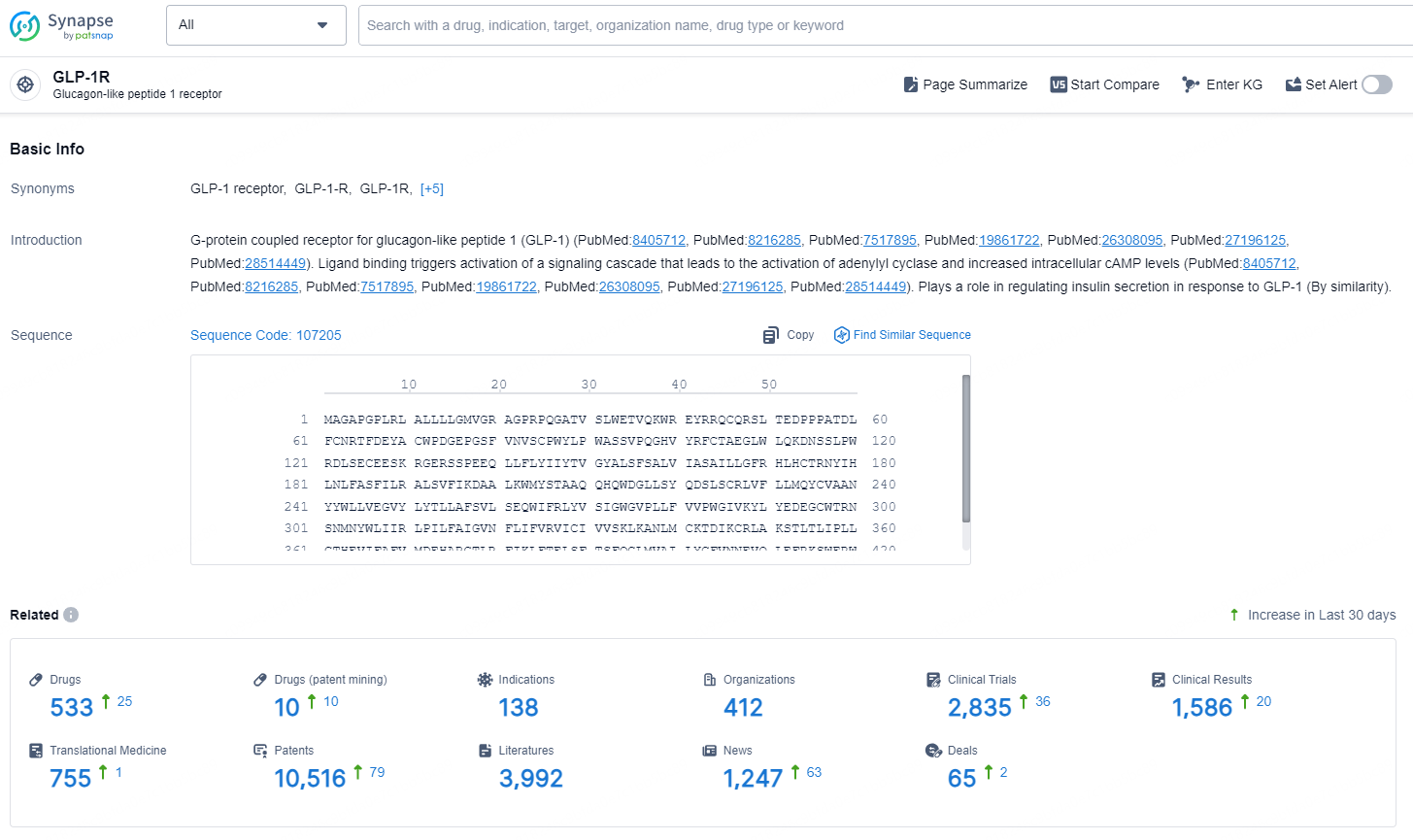
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 13, 2024, there are 533 investigational drugs for the GLP-1R target, including 138 indications, 412 R&D institutions involved, with related clinical trials reaching 2835, and as many as 10516 patents.
Bofanglutide is a biological product developed by Gan & Lee Pharmaceuticals Co., Ltd. The drug targets GLP-1R and is intended for the treatment of endocrinology and metabolic diseases, specifically Diabetes Mellitus, Type 2 and Obesity. The drug has reached Phase 2 in both the global and Chinese markets.