Fast-Track U.S. Approval Filed for Datopotamab Deruxtecan in Previously Treated Advanced EGFR-Mutated Non-Small Cell Lung Cancer
Daiichi Sankyo (TSE: 4568) and AstraZeneca (LSE/STO/Nasdaq: AZN) have filed a new Biologics License Application (BLA) seeking accelerated approval in the United States for datopotamab deruxtecan (Dato-DXd). This application is aimed at treating adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) that has mutations in the epidermal growth factor receptor (EGFR) and who have previously undergone systemic therapies, including treatment directed at EGFR.
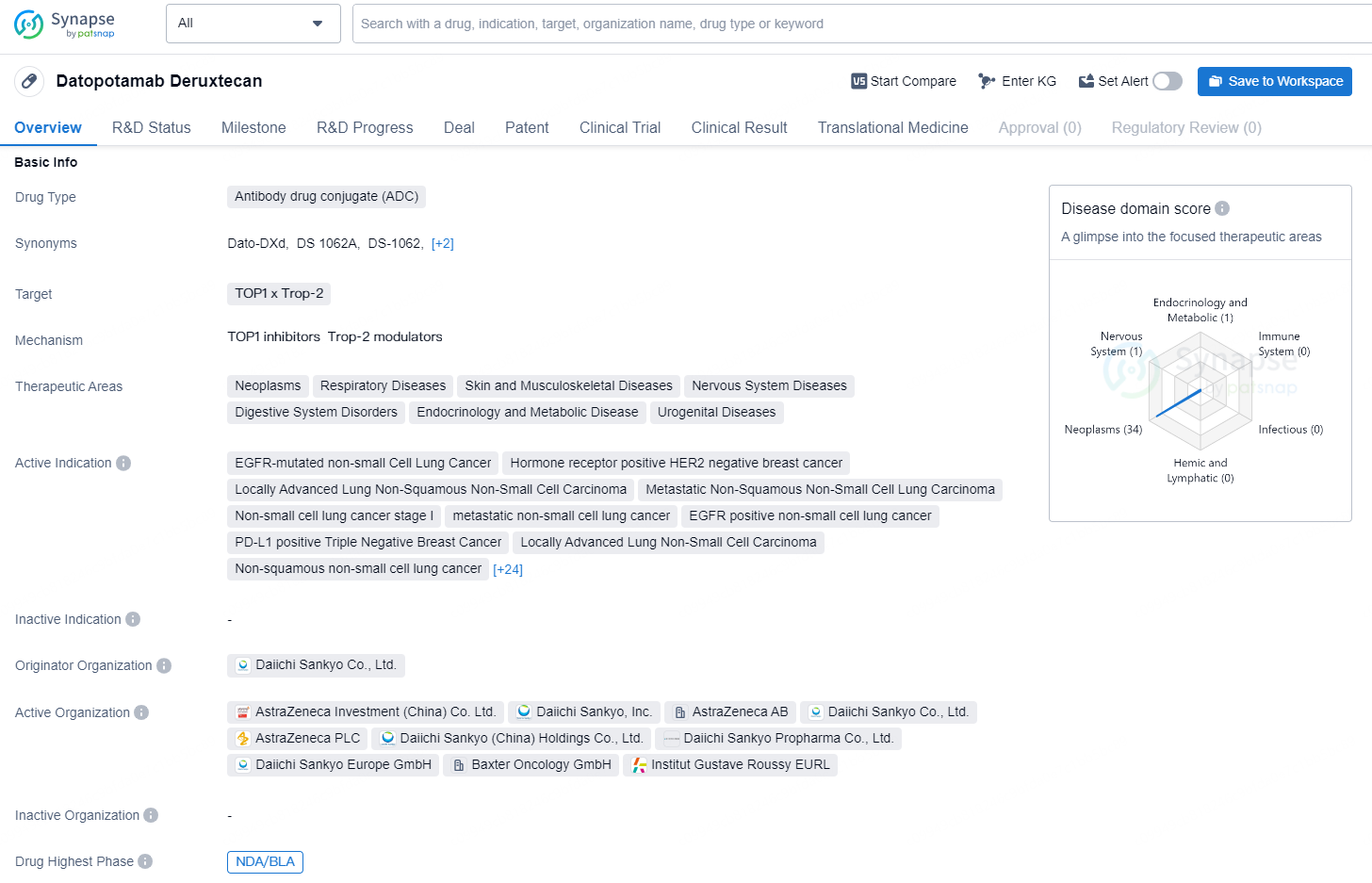
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The companies have voluntarily retracted the BLA in the United States for datopotamab deruxtecan concerning patients with advanced or metastatic nonsquamous NSCLC, following the results of the TROPION-Lung01 phase 3 trial.
This move to file a new BLA for EGFR-mutated NSCLC, as well as the withdrawal of the previously submitted application for nonsquamous NSCLC, was influenced by insights received from the U.S. Food and Drug Administration (FDA).
The forthcoming BLA is supported by findings from the TROPION-Lung05 phase 2 trial, alongside data from both the TROPION-Lung01 phase 3 trial and the TROPION-PanTumor01 phase 1 trial. Noteworthy findings from a pooled analysis of patients with previously treated EGFR-mutated NSCLC from the TROPION-Lung05 and TROPION-Lung01 studies will be presented in a late-breaking session at the upcoming European Society for Medical Oncology (ESMO) Asia 2024 Congress (LBA7).
Datopotamab deruxtecan is a specialized TROP2-directed DXd antibody-drug conjugate (ADC), discovered by Daiichi Sankyo and co-developed by Daiichi Sankyo and AstraZeneca.
"Addressing EGFR-mutated non-small cell lung cancer post-disease progression is significantly challenging due to the intricate nature and variability of these mutations, which frequently result in treatment resistance," noted Ken Takeshita, MD, Global Head of R&D at Daiichi Sankyo. "The potential approval of datopotamab deruxtecan may provide renewed optimism for individuals facing this difficult condition."
"Shooting for an improvement over standard chemotherapy for a wide-ranging, previously treated population with advanced lung cancer was the aim of TROPION-Lung01. The outcomes, along with the TROPION-Lung05 results, highlighted a notable advantage for patients with an EGFR mutation, which guided our talks with the FDA and our pursuit of accelerated approval for datopotamab deruxtecan in this demographic," stated Susan Galbraith, MBBChir, PhD, Executive Vice President of Oncology R&D at AstraZeneca. "Additionally, TROPION-Lung01 yielded promising exploratory data that underpins our biomarker development, set to be validated in ongoing and future phase 3 lung cancer trials."
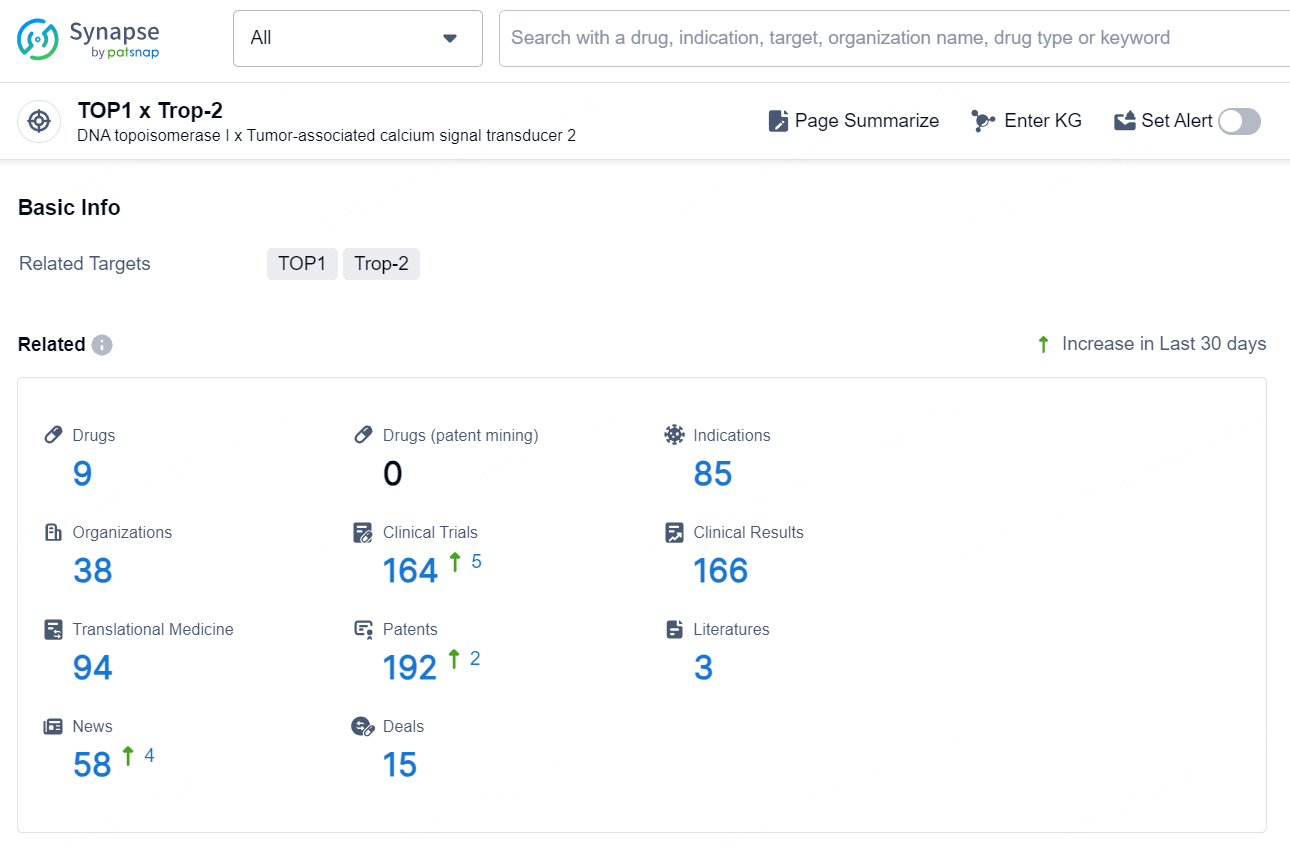
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of November 13, 2024, there are 9 investigational drugs for the TOP1 x Trop-2 target, including 85 indications, 38 R&D institutions involved, with related clinical trials reaching 164, and as many as 192 patents.
Datopotamab deruxtecan is an antibody drug conjugate (ADC) that targets TOP1 x Trop-2 and is being developed by Daiichi Sankyo Co., Ltd. The drug is intended for the treatment of a wide range of therapeutic areas, including neoplasms, respiratory diseases, skin and musculoskeletal diseases, nervous system diseases, digestive system disorders, endocrinology and metabolic disease, and urogenital diseases.