Omaveloxolone: Charting New Frontiers in Rare Disease Treatment and Beyond
Omaveloxolone is a small molecule drug developed by Reata Pharmaceuticals, Inc. that targets the Nrf2 protein. The drug has been approved for the treatment of Friedreich Ataxia, a rare genetic disease that causes progressive damage to the nervous system, resulting in movement problems and other symptoms. The drug has received approval in the United States, with the first approval date being in February 2023. It has been granted various regulatory designations, including Priority Review, Rare Pediatric Disease, Fast Track, and Orphan Drug status.
The approval of Omaveloxolone represents a significant milestone in the pharmaceutical industry, particularly in the development of treatments for rare and difficult-to-treat diseases. The drug's mechanism of action targeting the Nrf2 protein suggests potential benefits in addressing various diseases beyond Friedreich Ataxia, particularly those within the specified therapeutic areas.
Below, we will use the drug Omaveloxolone as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
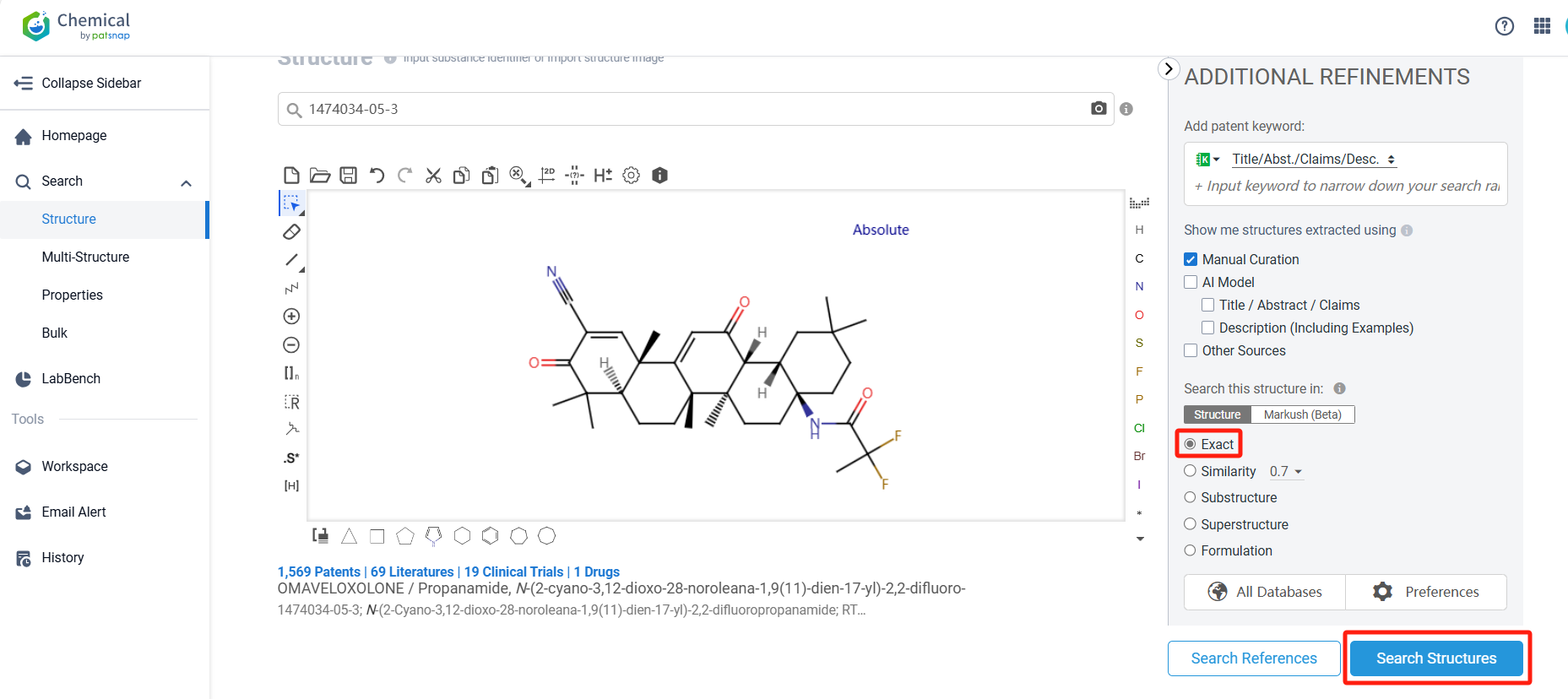
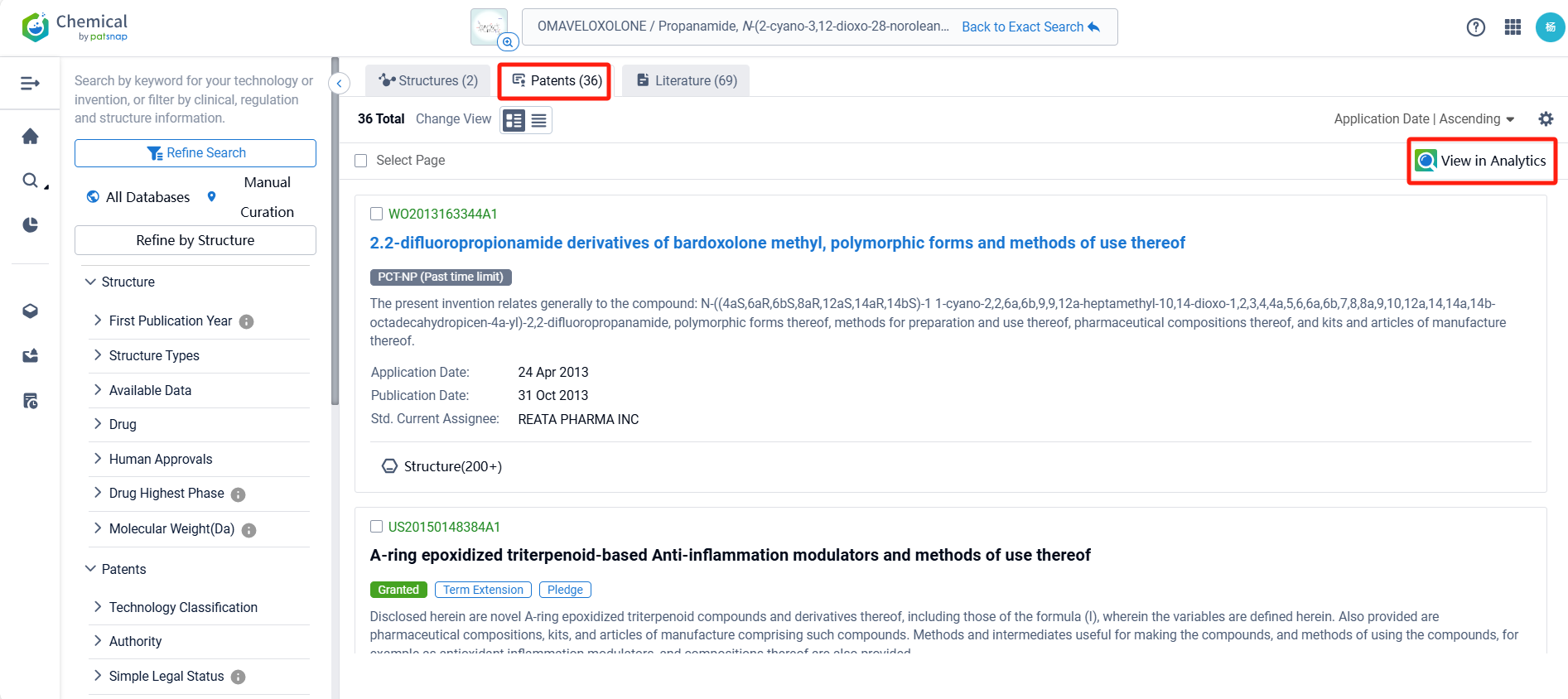
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Omaveloxolone (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, we select "Exact Search", click on search structures, and you can find 36 patents. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
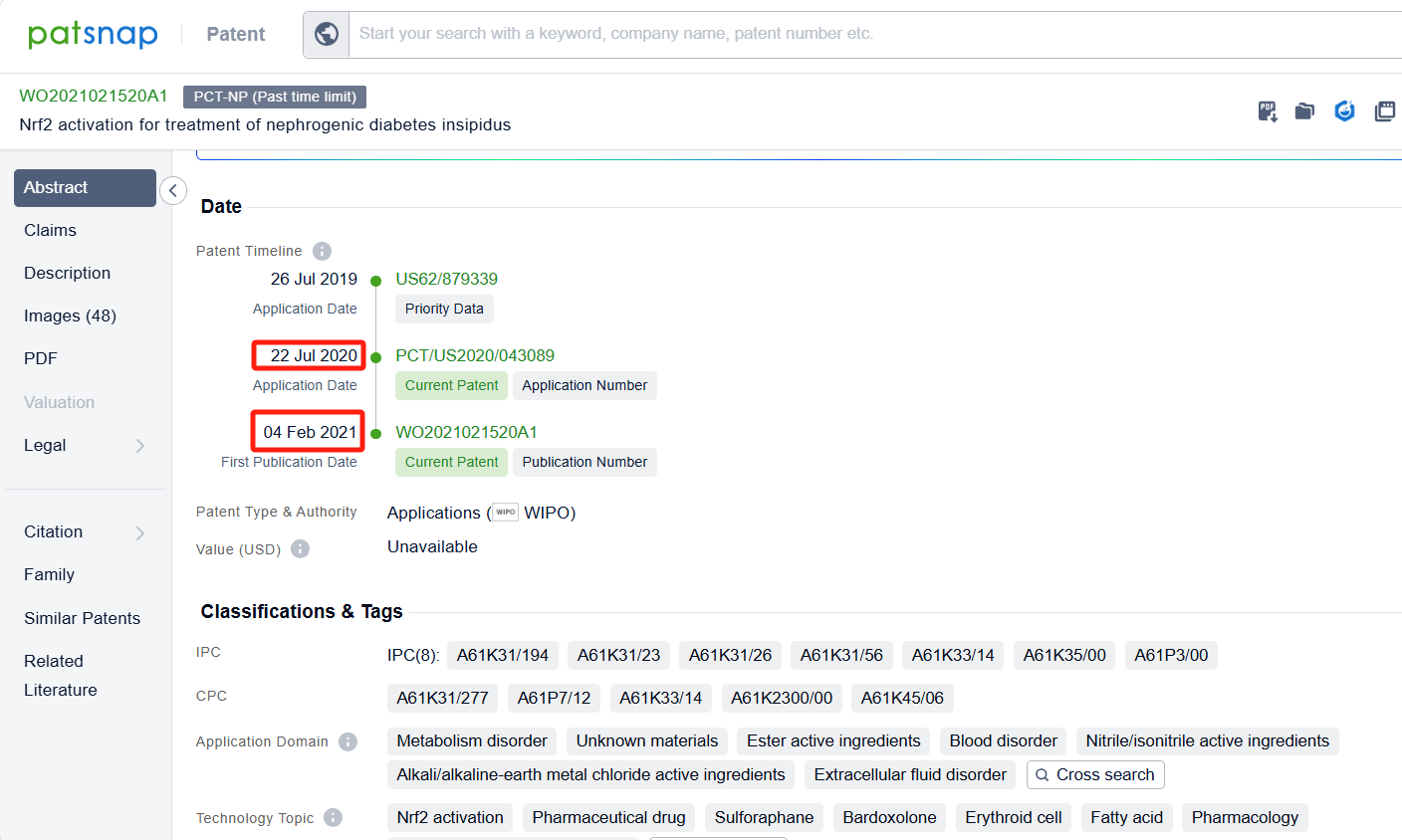
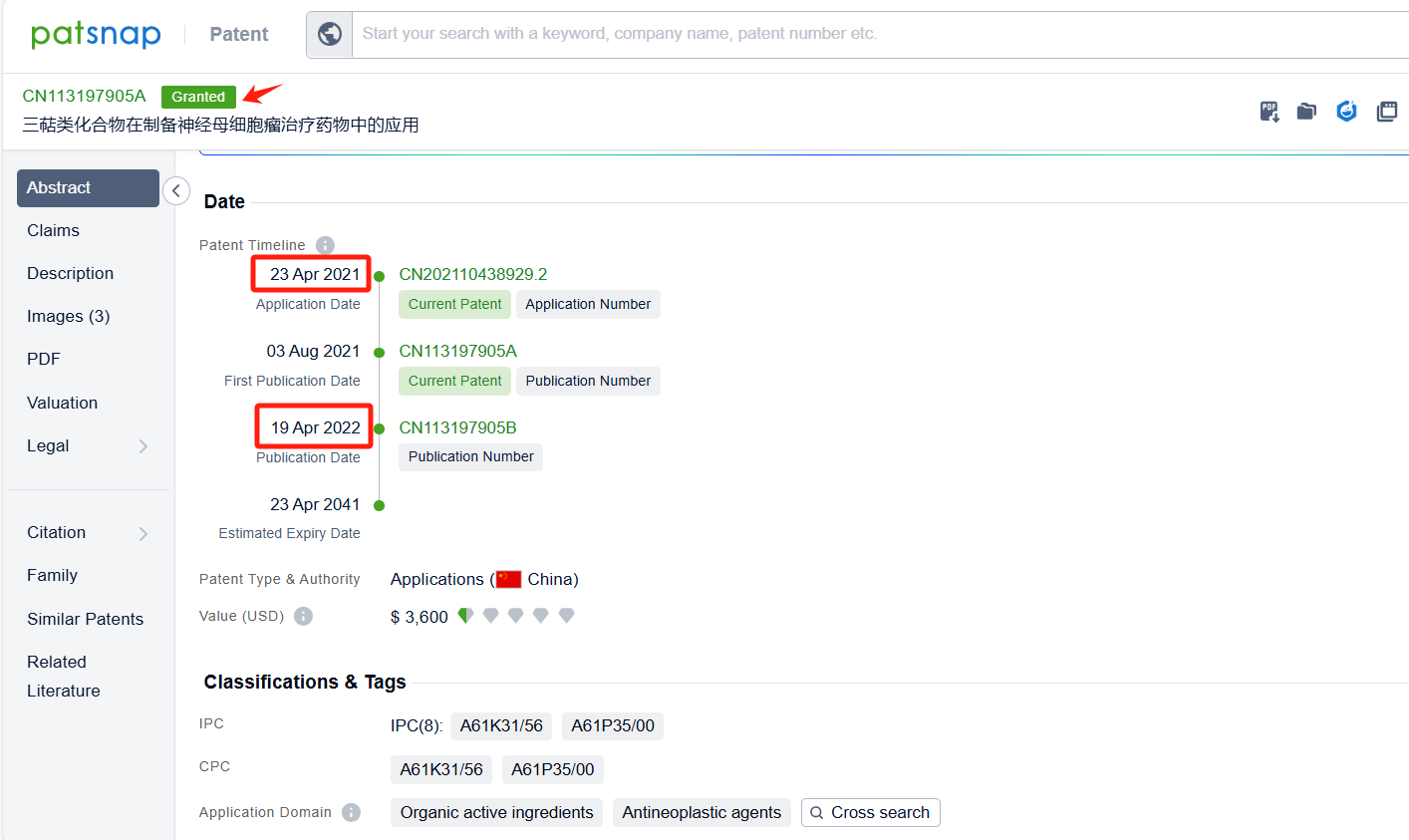
In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Omaveloxolone. By reviewing the aforementioned patents, we can observe that Univ of Pittsburgh 's international patent WO2021021520A1(application date 20200722, publication date 20210204) describes the use of a chemical called Nrf2 inducer to treat a condition called nephrogenic diabetes insipidus (NDI) caused by lithium. Additionally, Zhejiang University 's patent CN113197905A(application date 20210423, publication date 20210803) provides a novel compound RTA-408(Omaveloxolone), which can be used to treat neuroblastoma. This compound can improve the clinical efficacy of neuroblastoma patients and improve their prognosis and survival. The patent was granted in China on April 19, 2022.
The approval of Omaveloxolone could potentially pave the way for future developments in the treatment of Nrf2-related disorders and other diseases in the specified therapeutic areas. Additionally, the regulatory designations the drug has received indicate the recognition of its potential to address unmet medical needs, particularly in rare pediatric diseases, and the prioritization of its review process.
Overall, the approval of Omaveloxolone is a significant achievement for Reata Pharmaceuticals, Inc. and represents a promising advancement in the field of biomedicine, particularly in the treatment of rare diseases and conditions affecting the nervous system, congenital disorders, and endocrinology and metabolic diseases.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.