Pimicotinib Significantly Improves Outcomes in Phase III Study for Tenosynovial Giant Cell Tumor
Merck, a prominent company in the science and technology sector, has announced that the Phase III MANEUVER trial evaluating pimicotinib, an experimental drug under development by Abbisko Therapeutics Co., Ltd., has achieved its main objective. The trial showed a notable enhancement in the objective response rate (ORR) among individuals suffering from tenosynovial giant cell tumor (TGCT). At week 25, the ORR for those treated with pimicotinib reached 54.0%, in contrast to just 3.2% for the placebo group (p<0.0001).
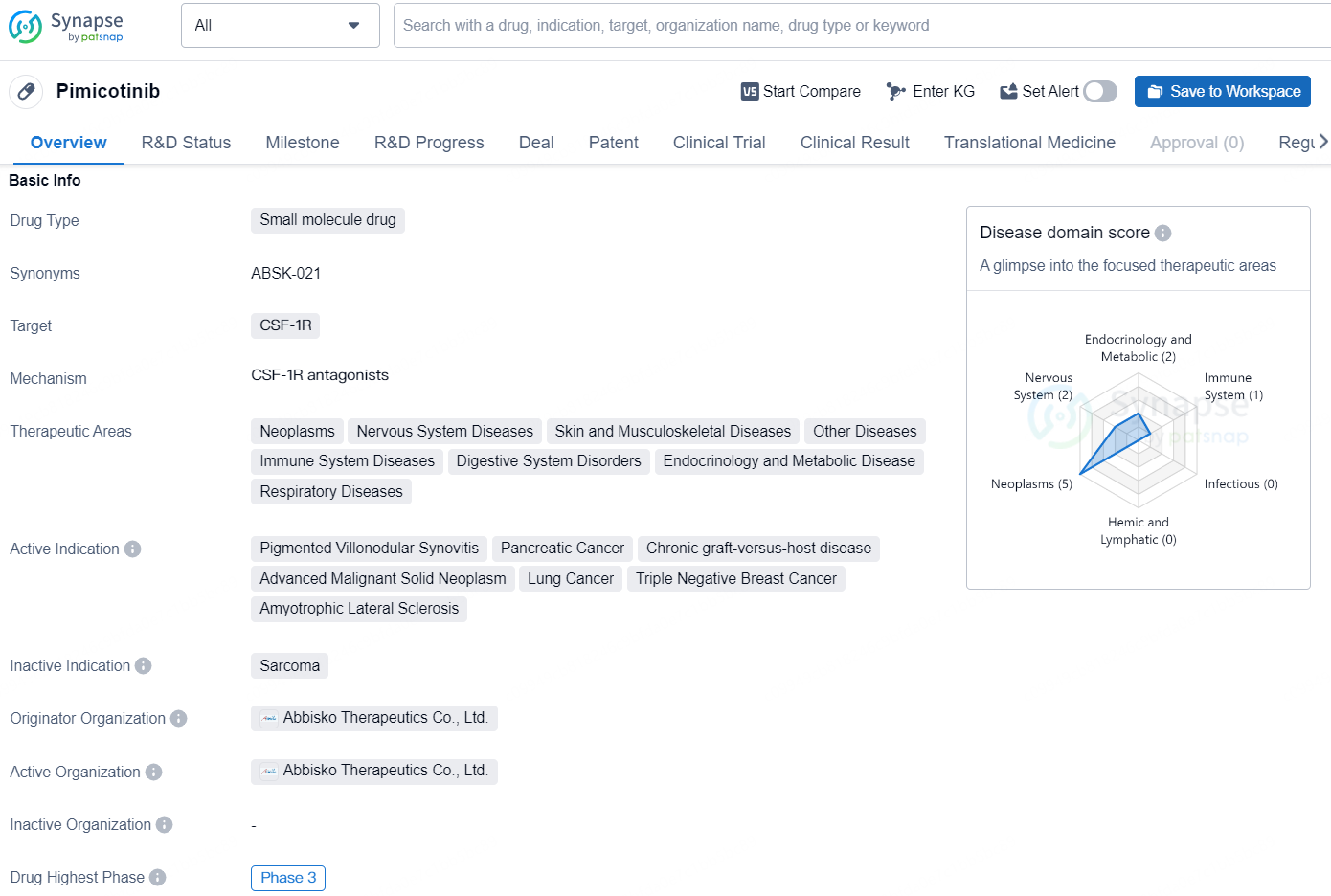
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The research revealed that pimicotinib treatment resulted in statistically significant and clinically relevant improvements in secondary endpoints related to key patient outcomes in TGCT. Notable findings included a mean change in stiffness measured by the Numeric Rating Scale (NRS) of -3.00 for the treatment group compared to -0.57 for the placebo group (p<0.0001) and a change in pain assessed by the Brief Pain Inventory (BPI) of -2.32 versus 0.23 for the placebo (p<0.0001). Additional efficacy and safety findings from the MANEUVER study are set to be revealed at an upcoming medical conference.
"TGCT is predominantly a disease affecting younger individuals. This uncommon, non-cancerous tumor that develops in and around joints primarily targets young and middle-aged adults during their employment years. The condition causes swelling, pain, stiffness, and restricted mobility, which can severely hinder daily activities, affecting both work and social engagements. Although surgery is a common treatment, high recurrence rates and possible complications from multiple surgeries can pose significant challenges, signaling an urgent need for systemic therapies that can manage tumor growth," explained Professor Niu Xiaohui, who leads the Bone and Soft Tissue Tumour Diagnosis and Research Centre at Beijing Jishuitan Hospital. "The findings from the MANEUVER study, along with the convenience of daily oral dosing that may enhance long-term compliance and pimicotinib’s targeted inhibition of CSF-1R, suggest that this investigational drug could pave the way for a novel treatment approach for TGCT patients."
In the MANEUVER trial, pimicotinib was found to be well-tolerated, with a safety profile aligning with earlier observations and no signs of cholestatic hepatotoxicity. Treatment-emergent adverse events (TEAEs) that led to discontinuation of therapy were reported in 1.6% (n=1) of subjects receiving pimicotinib, while TEAEs prompting a dose reduction occurred in 7.9% (n=5) of those treated with the medication.
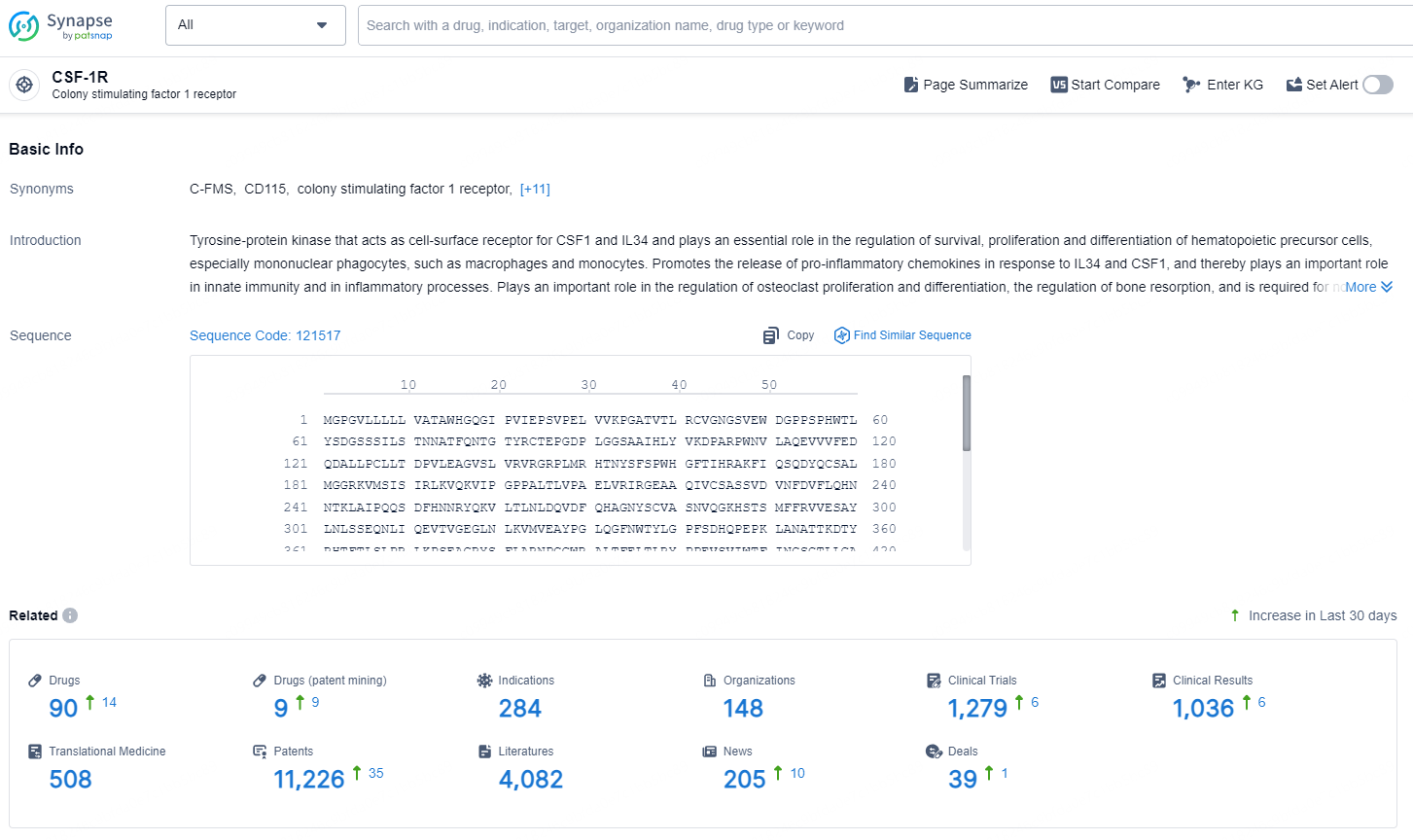
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 13, 2024, there are 90 investigational drugs for the CSF-1R target, including 284 indications, 148 R&D institutions involved, with related clinical trials reaching 2791, and as many as 11226 patents.
Pimicotinib is a small molecule drug that targets CSF-1R and has been developed by Abbisko Therapeutics Co., Ltd. The drug is currently in the highest phase of clinical development, Phase 3, both globally and in China.