Is Sitagliptin approved by the FDA?
Sitagliptin, marketed under the brand names Januvia and Zituvio, is approved by the U.S. Food and Drug Administration (FDA) for the treatment of type 2 diabetes mellitus in adults. This approval was first granted on October 18, 2006.
How Does Sitagliptin Work?
Sitagliptin belongs to a class of drugs known as dipeptidyl peptidase 4 (DPP-4) inhibitors. It works by regulating the levels of insulin your body produces after eating.
Dosage Information
The usual adult dose for type 2 diabetes is 100 mg taken orally once a day. This medication can be taken with or without food.
Common Side Effects
Some common side effects of sitagliptin include:
- Low blood sugar (hypoglycemia)
- Headache
- Runny or stuffy nose, sore throat
Serious Side Effects
Serious side effects can occur and require immediate medical attention:
- Signs of pancreatitis: severe pain in the upper stomach spreading to the back, with or without vomiting
- Severe autoimmune reactions: itching, blisters, breakdown of the outer layer of skin
- Severe joint pain
- Kidney issues: little or no urination
- Symptoms of heart failure: shortness of breath, swelling in legs or feet, rapid weight gain
Warnings and Precautions
Before taking sitagliptin, inform your doctor if you have any of the following conditions:
- Kidney disease (or if you are on dialysis)
- Heart problems
- Pancreatitis
- High triglycerides
- Gallstones
- Alcoholism
Sitagliptin is not recommended for use in individuals with diabetic ketoacidosis. Additionally, it may not be safe for use during pregnancy or breastfeeding. Consult your doctor for personalized advice.
Usage and Administration
- Follow the prescription label and medication guides.
- Your doctor may adjust your dose based on your response and blood sugar levels.
- Regular blood sugar tests are necessary to monitor effectiveness and adjust dosage if needed.
- Sitagliptin is part of a comprehensive treatment plan that includes diet, exercise, and possibly other medications.
Storage
Store sitagliptin tablets at room temperature, away from moisture, heat, and light.
Conclusion
Sitagliptin (Januvia, Zituvio) is FDA-approved for managing type 2 diabetes in adults. If you are considering or already taking sitagliptin, it's important to follow your doctor's guidelines and report any unusual symptoms or side effects immediately.
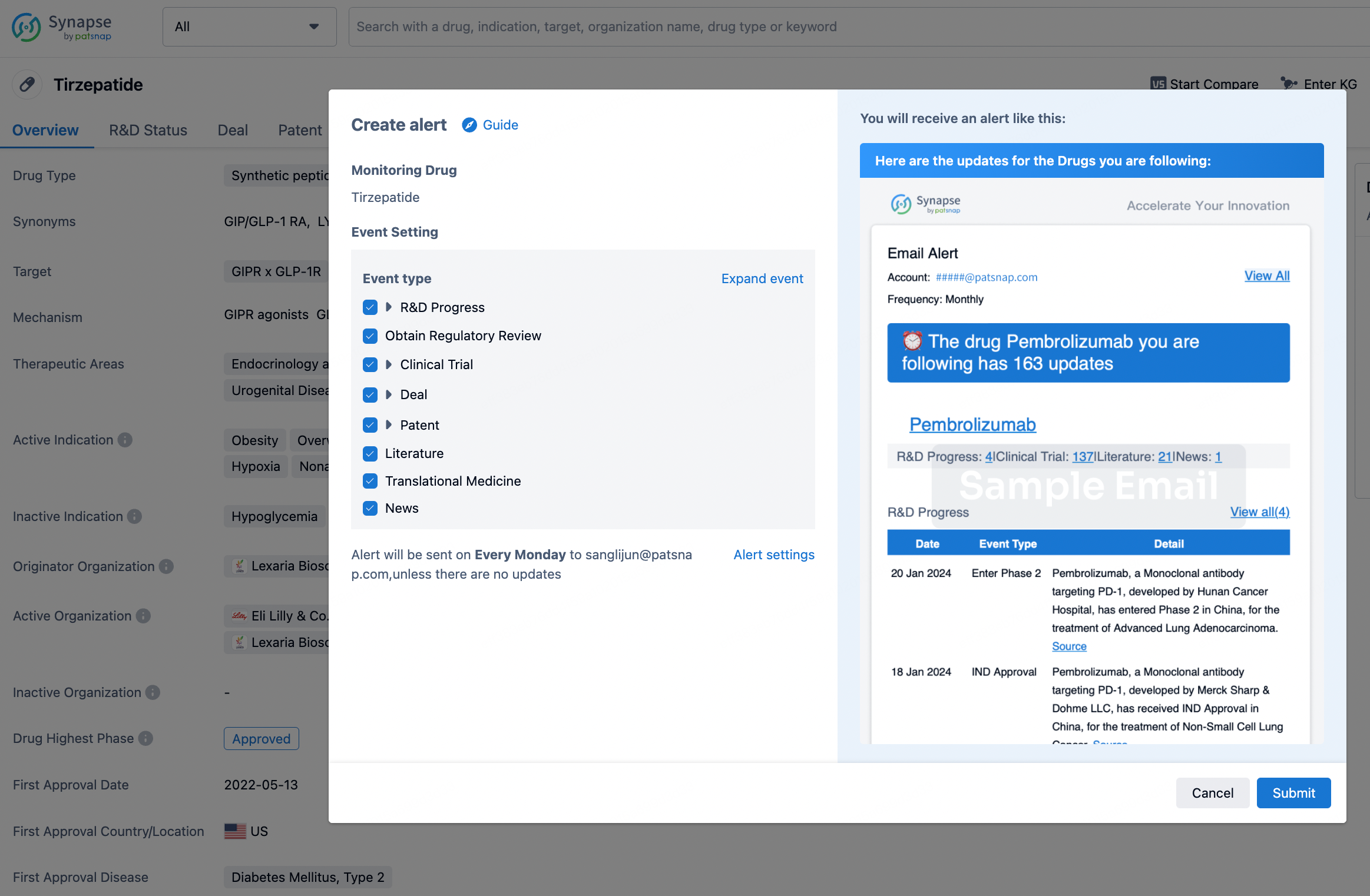
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!