Eli Lilly's $660 Million Collaboration and Notch Pathway Signal Inhibitors
Eli Lilly currently holds the throne as the world's most valuable pharmaceutical company by market capitalization. On July 15, 2024, Eli Lilly's stock price reached a historical high of $966.1 per share, with a company valuation of $910 billion, getting closer to becoming the first trillion-dollar valued pharmaceutical company. Eli Lilly's financial report for 2023 shows that its revenue mainly comes from dulaglutide ($7.133 billion), tirzepatide ($5.163 billion), abemaciclib ($3.863 billion), and ixekizumab ($2.76 billion), among others. With the continued volume growth of tirzepatide and the market launch of the new Alzheimer's drug Donanemab, the future remains promising.
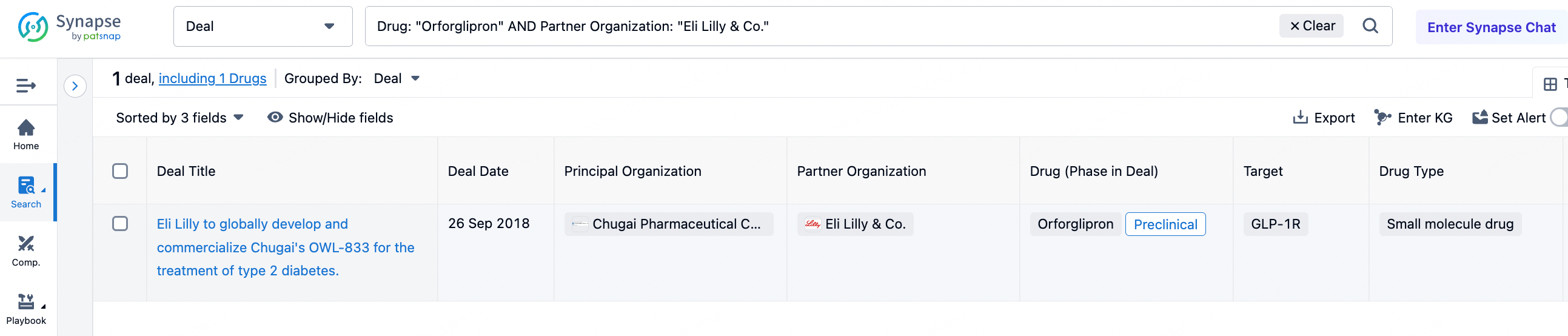
Eli Lilly's success is closely related to its inherent pragmatism. Although many of its products are not the first to be approved and launched, Eli Lilly's drugs are always present in popular targets and competitive fields, such as CDK4/6, IL-17, IL-23, and GLP-1. Eli Lilly's acquisitions are also discerning; for example, in September 2018, it acquired the GLP1 small molecule agonist Orforglipron from the Japanese company Chugai, which was still in the preclinical stage at the time.
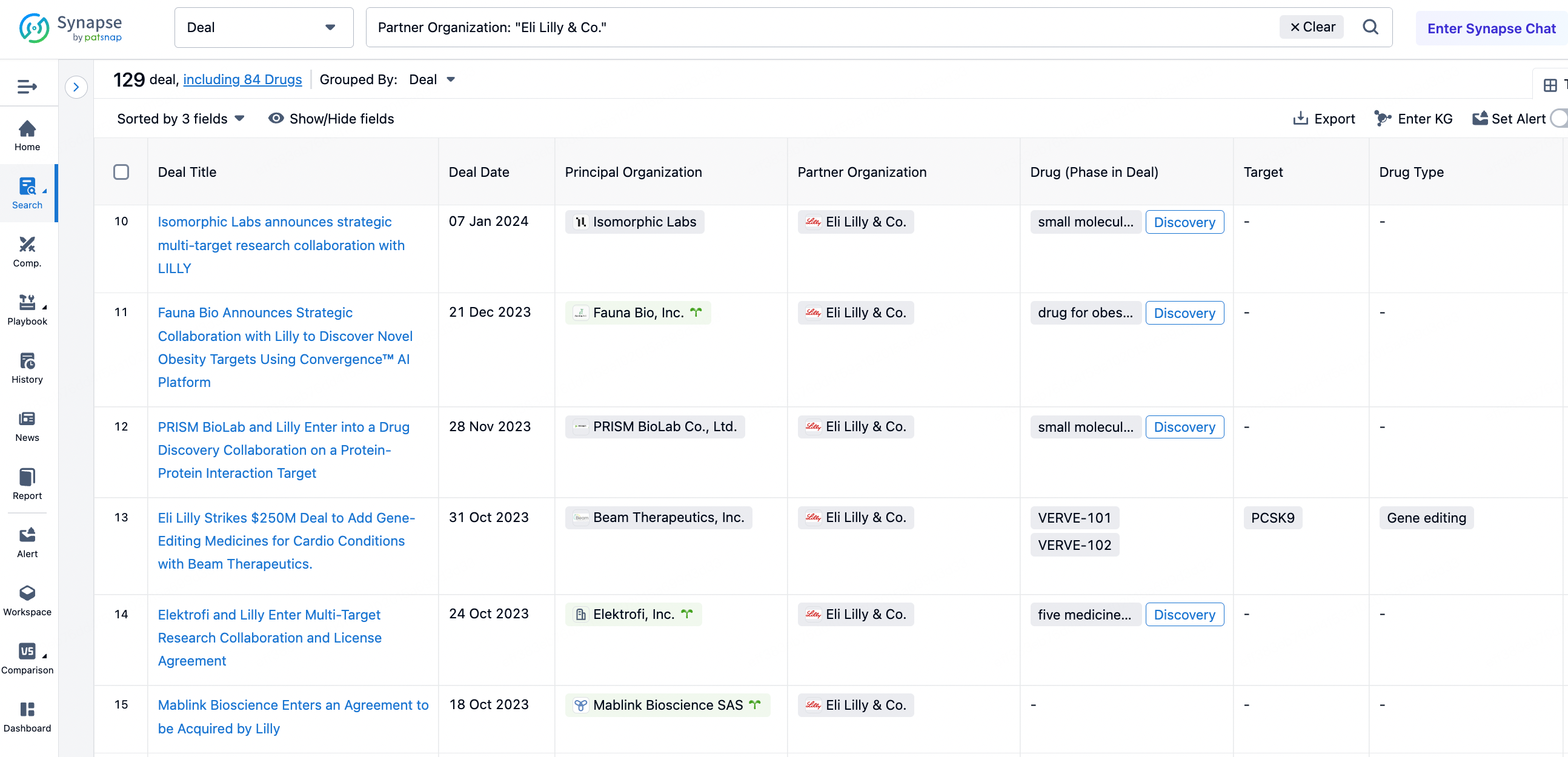
As Pfizer announced the discontinuation of the development of lotiglipron due to liver toxicity issues, Eli Lilly's Orforglipron has taken the lead globally, advancing to Phase 3 first. Compared to Eli Lilly's various successful acquisition cases, Gilead's acquisitions outside the viral field have almost entirely failed. For R&D personnel, this may serve as a guide to avoiding pitfalls. In the synapse database's transaction section, by selecting Eli Lilly as the transferee, one can see 129 transaction records. Among them, the transaction with PRISM BioLab on November 28, 2023, caught the author's attention.
This licensing and cooperation agreement, which may total up to $660 million, aims to jointly develop oral small molecule drugs. PRISM BioLab utilizes its proprietary PepMetics technology to discover orally available small molecule inhibitors targeting protein-protein interaction (PPI) pathways, to treat patients with cancer, autoimmune, fibrotic, and other diseases. PepMetics are a unique class of small molecules that mimic the three-dimensional structures of α-helices and β-turns, which are common peptide structures in cellular PPIs and receptor-ligand interactions. According to the agreement, the two companies will collaborate to discover small molecule inhibitors of PPI targets selected by Eli Lilly through PRISM's proprietary PepMetics technology. Eli Lilly has the option to add two more targets to the collaboration and is responsible for the clinical development and commercialization of the resulting products. Although PRISM has already established cooperation with large pharmaceutical companies such as Eisai, Boehringer Ingelheim, Merck KGaA, and Roche, no intelligence information related to MNC companies has been found in either the pipeline or patent applications.
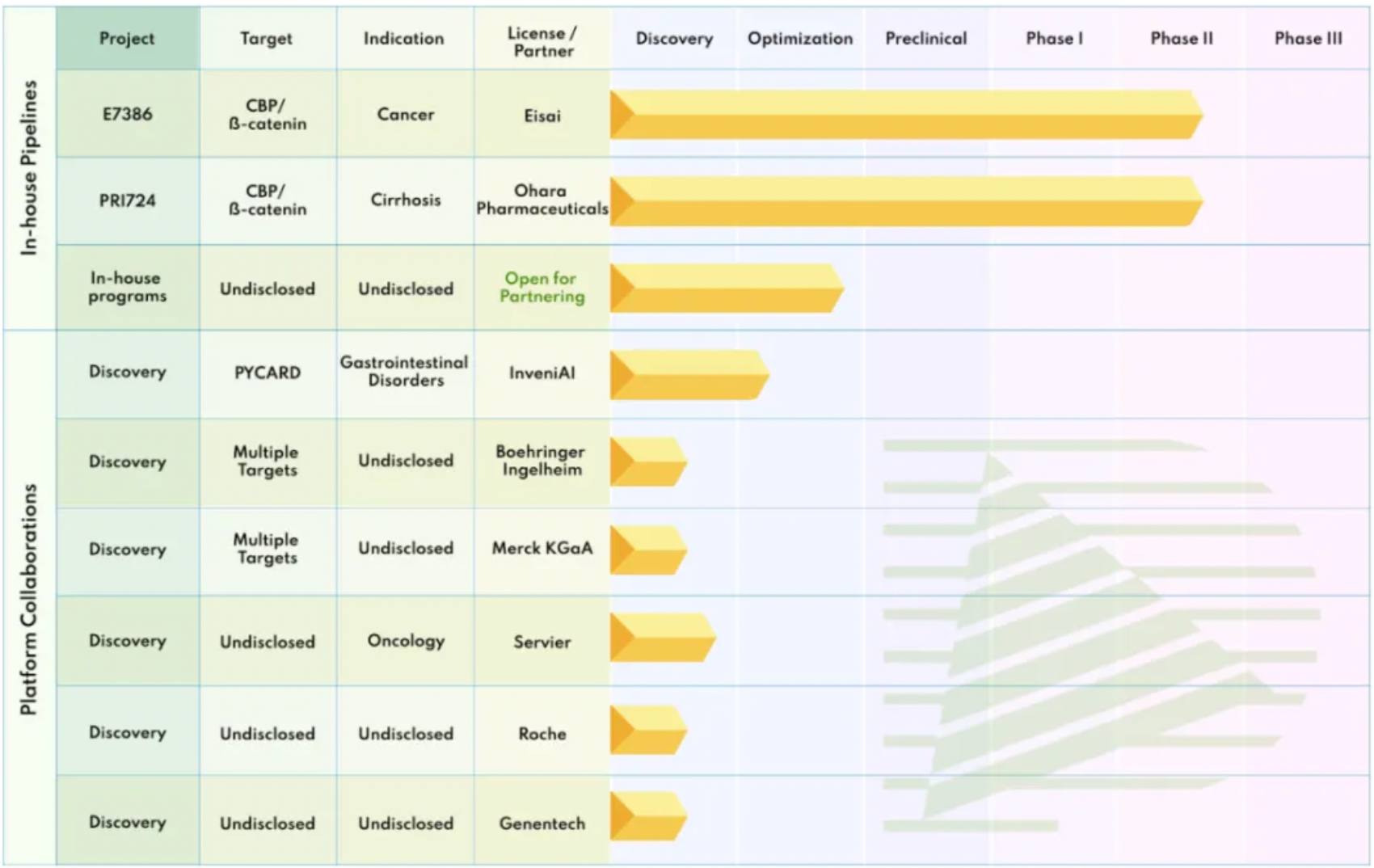
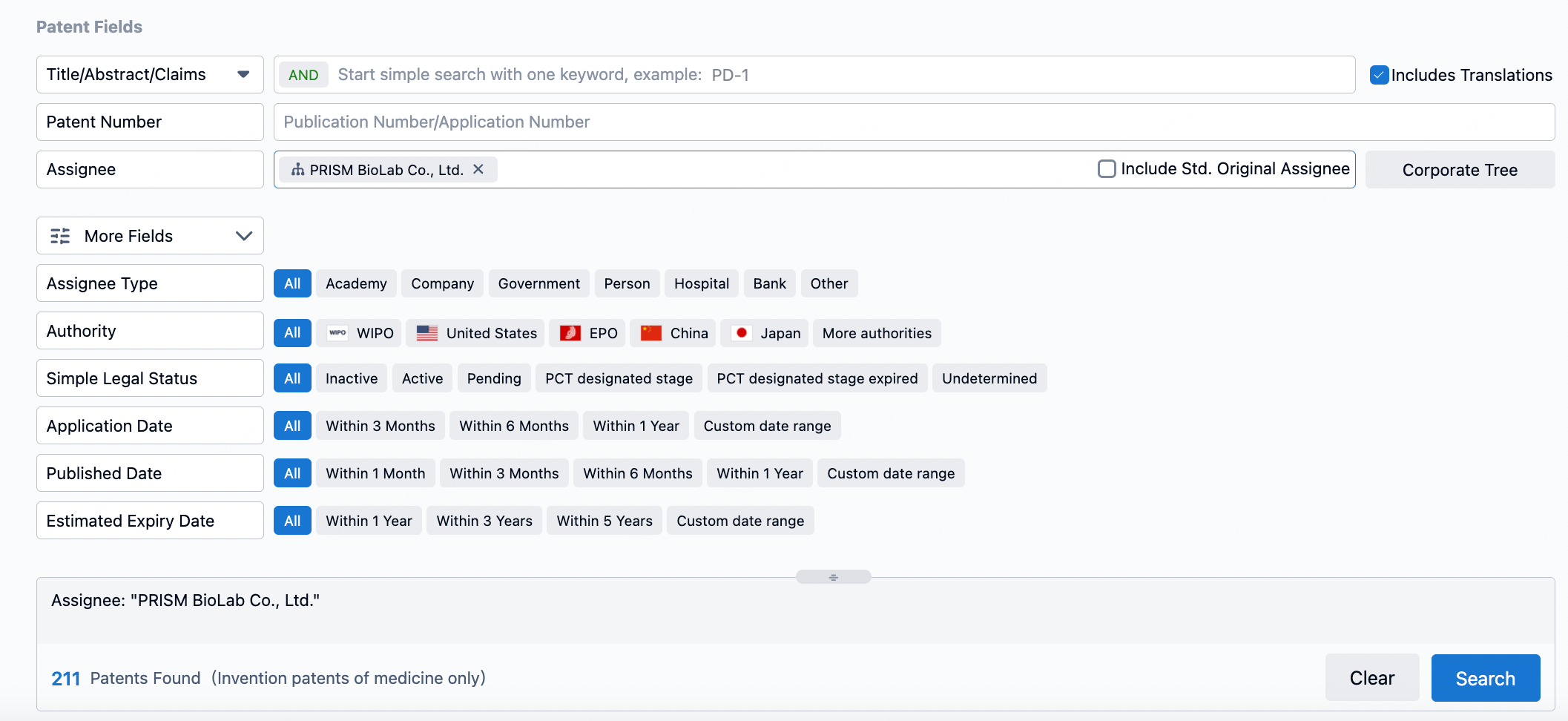
In the patent section of the Synapse database, searching for patents applied for by PRISM BioLab yields over 211 entries. By limiting the publication date to the most recent five-year period, one can get a general sense of the company's research and development activities. The first six patents published include WO2023200017, WO2023140369, WO2023090366, WO2022158610, WO2022075486, and WO2022014724; half of these patents are related to inhibitors of the Notch signaling pathway.
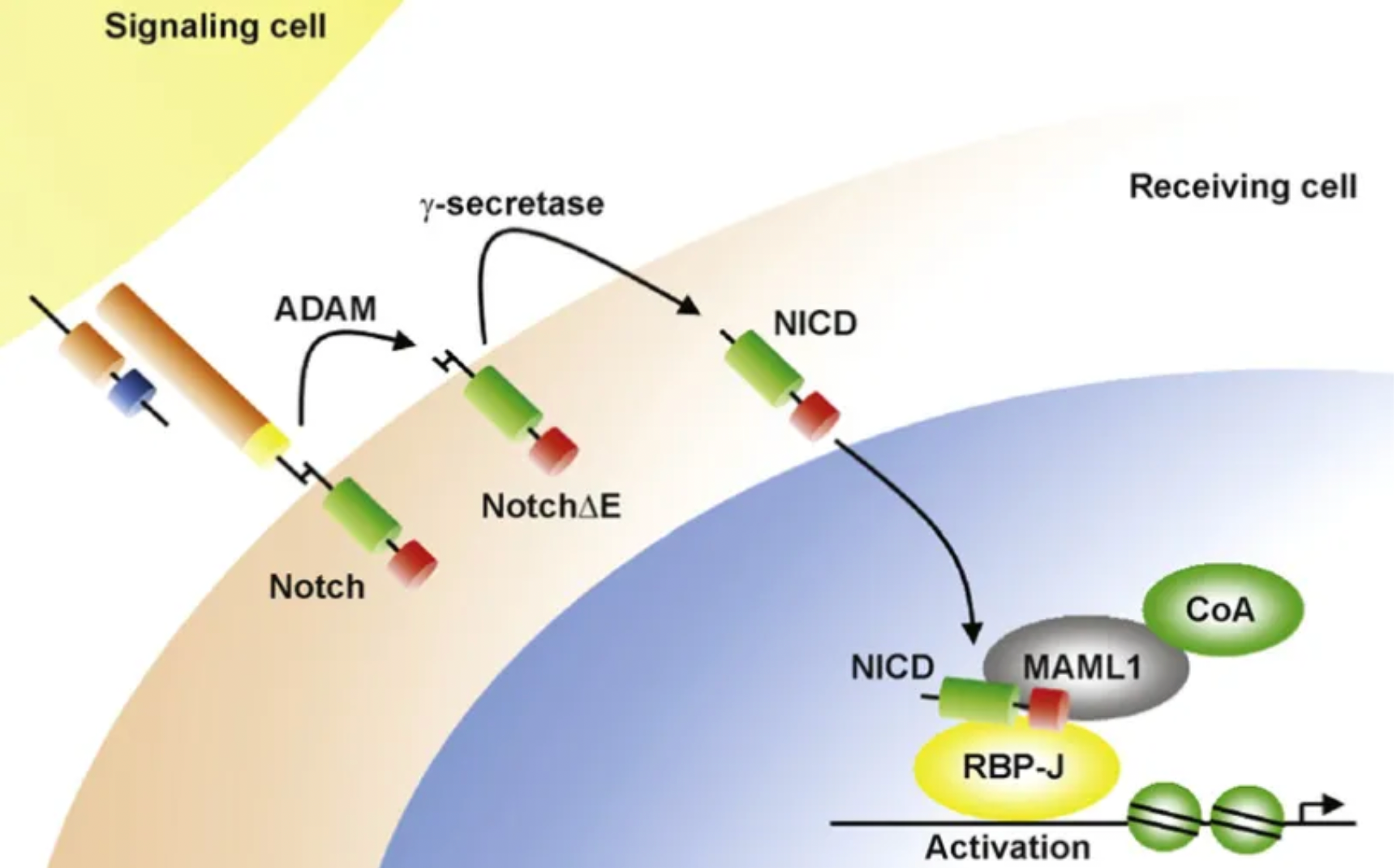
The Notch signaling pathway is an evolutionarily conserved mechanism that plays an indispensable role in the development and tissue homeostasis of mammals. Notch receptors and ligands contain single-pass transmembrane domains and are expressed on the cell surface; therefore, Notch signaling is particularly important in mediating communication between adjacent cells that express the receptors and ligands.
In rodents and humans, four known Notch receptors have been identified, termed Notch 1 to Notch 4. Notch receptors are heterodimeric proteins composed of extracellular and intracellular domains that are initially synthesized as a single polypeptide. The receptor-ligand interaction triggers a series of proteolytic cleavages of the Notch receptor polypeptide, involving the activity of γ-secretase. The activity of γ-secretase cleaves the Notch intracellular domain (NICD) on the inner side of the plasma membrane, which then translocates to the nucleus to form a transcription factor complex.
The Notch intracellular domain (NICD) is the active form of the protein. Various functions of Notch signaling include proliferation, differentiation, apoptosis, angiogenesis, migration, and self-renewal. Additionally, NICD activates the transcription of target genes Hes1 and Hes5 by translocating to the nucleus and forming a stable complex with RBP-J and MAML, which act as DNA-binding proteins. Therefore, compounds that can inhibit various functions of the Notch signaling pathway have the potential to become drugs for a variety of diseases involving these functions.
A series of therapeutic strategies targeting the abnormalities of the Notch signaling pathway are currently under investigation and development. For instance, inhibitors of the Notch signaling pathway, such as γ-secretase inhibitors, are being studied and clinically trialed as potential treatment methods for acute lymphoblastic leukemia (ALL) and T-cell lymphoma (T-ALL).
Based on the current state of Notch pathway inhibitors in clinical stages, the field is not yet crowded and is at a stage of interest to MNC companies. Eli Lilly and BMS show a high level of interest in this area. Eli Lilly has two molecules in clinical development: the Phase I LY3039478 and the discontinued LY900009. Eli Lilly's recent acquisition may also be related to collaboration on these pathway inhibitors. After all, PRISM BioLab's recent patents include three that belong to Notch inhibitors, with numerous examples in the patents and key molecules featuring more than three chiral centers. The most recently published patent, WO2023200017, has evolved from a bicyclic to a tricyclic heptamer, indicating that the synthesized molecules are becoming increasingly complex, which also demonstrates PRISM BioLab's significant focus on the Notch pathway.
At present, drugs targeting the Notch signaling pathway are still under research. The Notch signaling pathway plays a crucial role in a variety of physiological processes, and intervention must be approached with caution to avoid severe toxic side effects. The regulatory mechanism of the Notch signaling pathway is complex, and how to precisely control it to achieve therapeutic effects remains an important direction for future research.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!