Sandoz Launches Tyruko®, a Unique Biosimilar for Multiple Sclerosis Treatment, in Germany
Sandoz, an internationally recognized pioneer in the production of generics and biosimilars, has revealed the introduction of its product, Tyruko® (natalizumab), in the German market starting February 1. Originating from the research and development efforts of Polpharma Biologics, Tyruko® stands out as the inaugural biosimilar on the market specifically formulated for the management of Relapsing-Remitting Multiple Sclerosis (RRMS).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.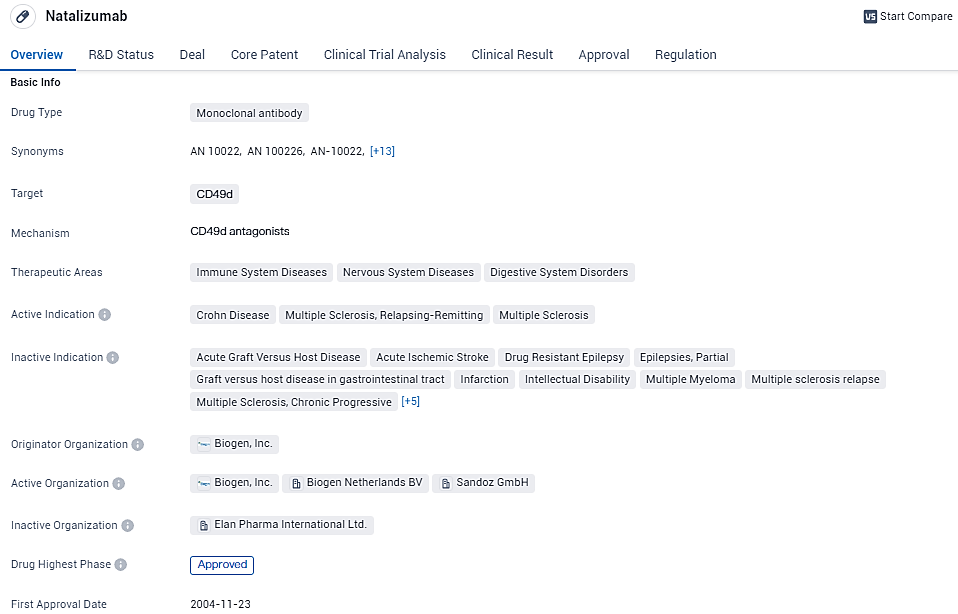
Tyruko® is recommended for sole use as a transformative treatment for adult individuals battling severe forms of RRMS. This usage is congruent with the prior authorization granted by the European Commission for the originator medicine, Tysabri®.
Rebecca Guntern, who leads the European division for Sandoz, commented, “Initiating treatment with transformative therapies early on can greatly influence the lives of those affected by multiple sclerosis, potentially curbing future physical impairments. Tyruko®, being the unique biosimilar of its kind, marks a pivotal point in extending the reach of efficacious and reliable treatments to individuals across Europe, particularly to those whose needs are most pressing.”
Currently, only about 20% of patients with MS in Europe have the opportunity to benefit from cutting-edge, highly potent DMTs. In Eastern Europe, the percentage is drastically lower, between 3% and 4%, indicating a pressing need to expand uninhibited access to these vital medications to delay irreversible neurological harm and halt the progression of the disease.
In 2019, Sandoz secured a comprehensive licensing deal with Polpharma Biologics for the global commercialization of a biosimilar natalizumab. As per the terms, Polpharma Biologics is tasked with the continued development, manufacturing, and provision of the active pharmaceutical ingredient. Sandoz, through an exclusive worldwide license, is authorized to market and disseminate the drug across international territories.
Sandoz pledges to facilitate access to essential biologic drugs for millions of patients at sustainable and economical prices, impacting diverse medical fields such as immunology, oncology, supportive care, endocrinology, and now includes neurology as well. It boasts a prominent global portfolio of nine biosimilars that have already hit the market, along with an additional 24 in the pipeline.
Since the introduction of its premier biosimilar in Europe back in 2006, Sandoz has consistently worked towards enhancing patient access to transformative drugs earlier, fostering economic healthcare solutions, and stimulating competitiveness that drives further medical advancements.
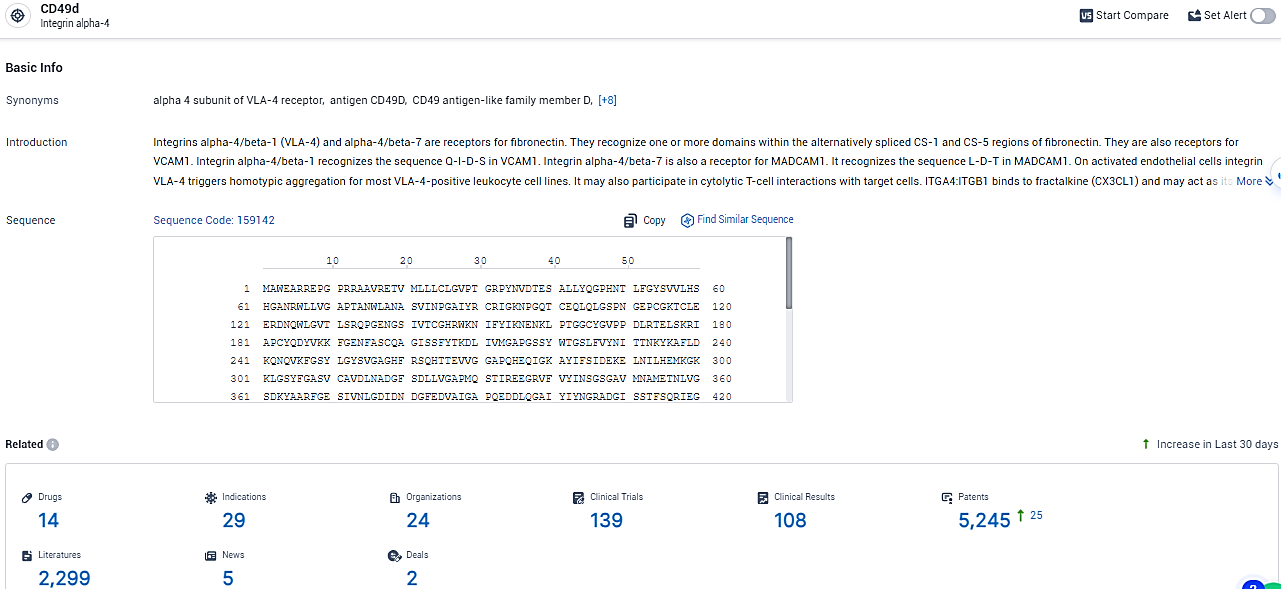
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 7, 2024, there are 14 investigational drugs for the CD49d target, including 29 indications, 24 R&D institutions involved, with related clinical trials reaching 139, and as many as 5245 patents.
Natalizumab targets CD49d and is approved for the treatment of Crohn's disease and relapsing-remitting MS. The drug has received accelerated approval and orphan drug designation, reflecting its potential to address unmet medical needs. While Natalizumab has shown efficacy in clinical trials, it is important to consider the associated risks and carefully evaluate its use in patients.