FDA Approves Autolus Therapeutics' AUCATZYL® for Adult Relapsed or Refractory B-cell Acute Lymphoblastic Leukemia
Autolus Therapeutics plc (Nasdaq: AUTL), a biopharmaceutical firm in its early commercial phase specializing in advanced programmed T cell therapies, has reported that the U.S. Food and Drug Administration (FDA) has issued marketing authorization for AUCATZYL® (obecabtagene autoleucel) aimed at treating adult patients suffering from relapsed or refractory B-cell precursor acute lymphoblastic leukemia (r/r B-ALL).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
“Adult acute lymphoblastic leukemia (ALL) is a highly aggressive form of cancer, and there is a significant unmet medical need for effective treatment options for patients who experience a relapse, historically resulting in unfavorable outcomes,” remarked Dr. Elias Jabbour, principal investigator for the FELIX study and a professor at The University of Texas MD Anderson Cancer Center, Houston, Texas. “The recent approval of AUCATZYL, which showcased its clinical benefit, offers renewed hope for adults battling relapsed/refractory B-ALL.”
The FDA granted approval for AUCATZYL following outcomes from the FELIX clinical trial involving obe-cel in adults with relapsed/refractory B-ALL. Among the morphological disease cohort, 94 participants received at least one dose of AUCATZYL. Out of these, 65 patients exhibited over 5% blasts in their bone marrow at the time of screening and before the lymphodepletion phase, making them eligible for efficacy evaluation. Among the efficacy-evaluable cohort (n=65), 63% attained overall complete remission (OCR1), which included 51% of patients achieving complete remission (CR) at any point and 12% achieving complete remission with incomplete blood count recovery (CRi) at any time.
The primary efficacy endpoint was complete remission within three months, observed in 42% of the patients, with a median duration of remission (DOR) reported at 14.1 months. AUCATZYL was associated with low occurrences of Cytokine Release Syndrome (CRS), with 3% of patients experiencing Grade 3 events, while no Grade 4 or 5 events were noted. Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) at Grade ≥ 3 was documented in 7% of participants. Additionally, the FDA did not mandate a Risk Evaluation and Mitigation Strategy (REMS) for AUCATZYL.
The safety profile of AUCATZYL includes a boxed warning for potential CRS, neurological toxic effects, and the risk of secondary hematological malignancies. ICANS, which may include severe or potentially fatal adverse reactions, were noted in patients treated with AUCATZYL. There is a risk of developing T-cell malignancies post-treatment with BCMA- and CD19-targeting genetically engineered autologous T-cell therapies in patients who have undergone treatment for hematologic malignancies. According to the FELIX trial, the most frequently reported non-laboratory adverse reactions (with an incidence of ≥ 20%) included CRS, infections of unspecified origin, musculoskeletal pain, viral infections, fever, nausea, bacterial infections, diarrhea, febrile neutropenia, ICANS, hypotension, pain, fatigue, headache, encephalopathy, and hemorrhagic events.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
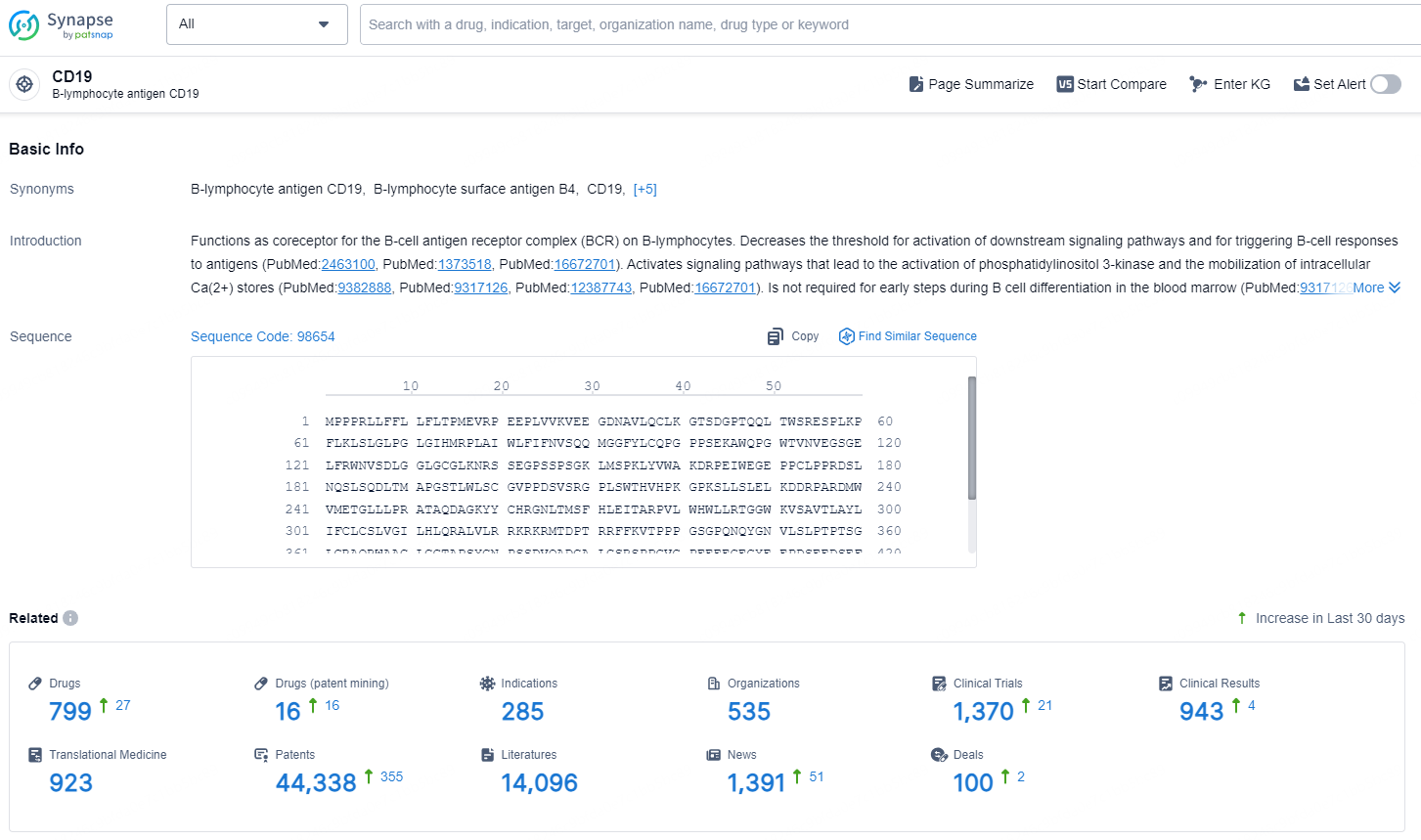
According to the data provided by the Synapse Database, As of November 13, 2024, there are 799 investigational drugs for the CD19 target, including 285 indication, 535 R&D institutions involved, with related clinical trial reaching 1370, and as many as 44338 patents.
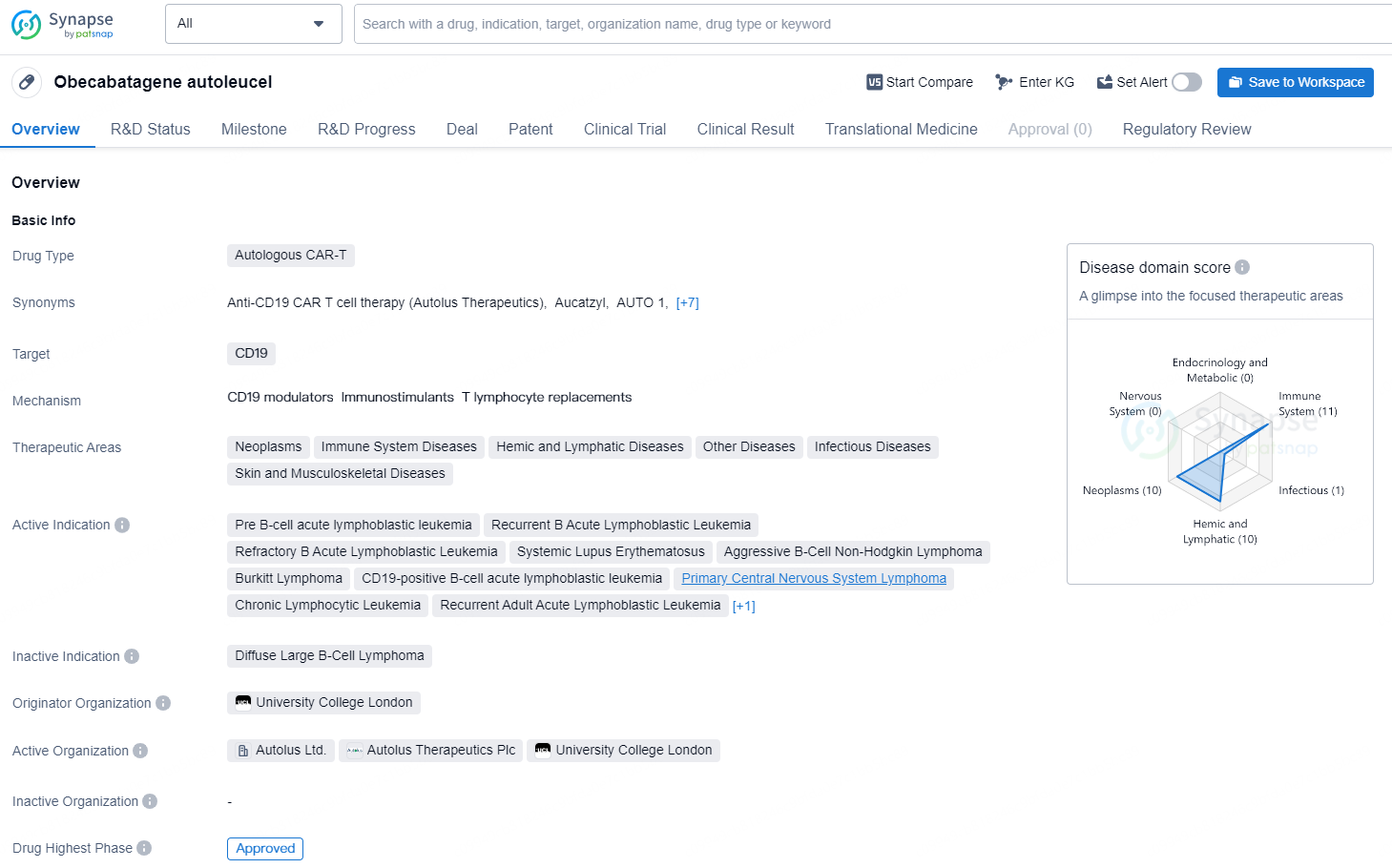
Obecabtagene autoleucel is an autologous CAR-T (chimeric antigen receptor T-cell) therapy that targets CD19 in various therapeutic areas, including neoplasms, immune system diseases, hemic and lymphatic diseases, infectious diseases, and skin and musculoskeletal diseases. The drug is indicated for the treatment of a range of conditions, including pre B-cell acute lymphoblastic leukemia, recurrent and refractory B acute lymphoblastic leukemia, systemic lupus erythematosus, aggressive B-cell non-Hodgkin lymphoma, Burkitt lymphoma, primary central nervous system lymphoma, and chronic lymphocytic leukemia.