STORM Therapeutics Unveils Fresh Clinical Findings on METTL3 Inhibitor STC-15 at SITC 2024
STORM Therapeutics Ltd. (STORM), a company at the clinical stage focused on innovative cellular reprogramming using RNA modifications for disease treatment, reveals that it shared encouraging findings from its Phase 1 clinical trial of STC-15, an inhibitor targeting METTL3 RNA methyltransferase. This presentation took place at the 39th Annual Meeting of the Society of Immunotherapy for Cancer (SITC) in Houston, US, from November 6-10, 2024.
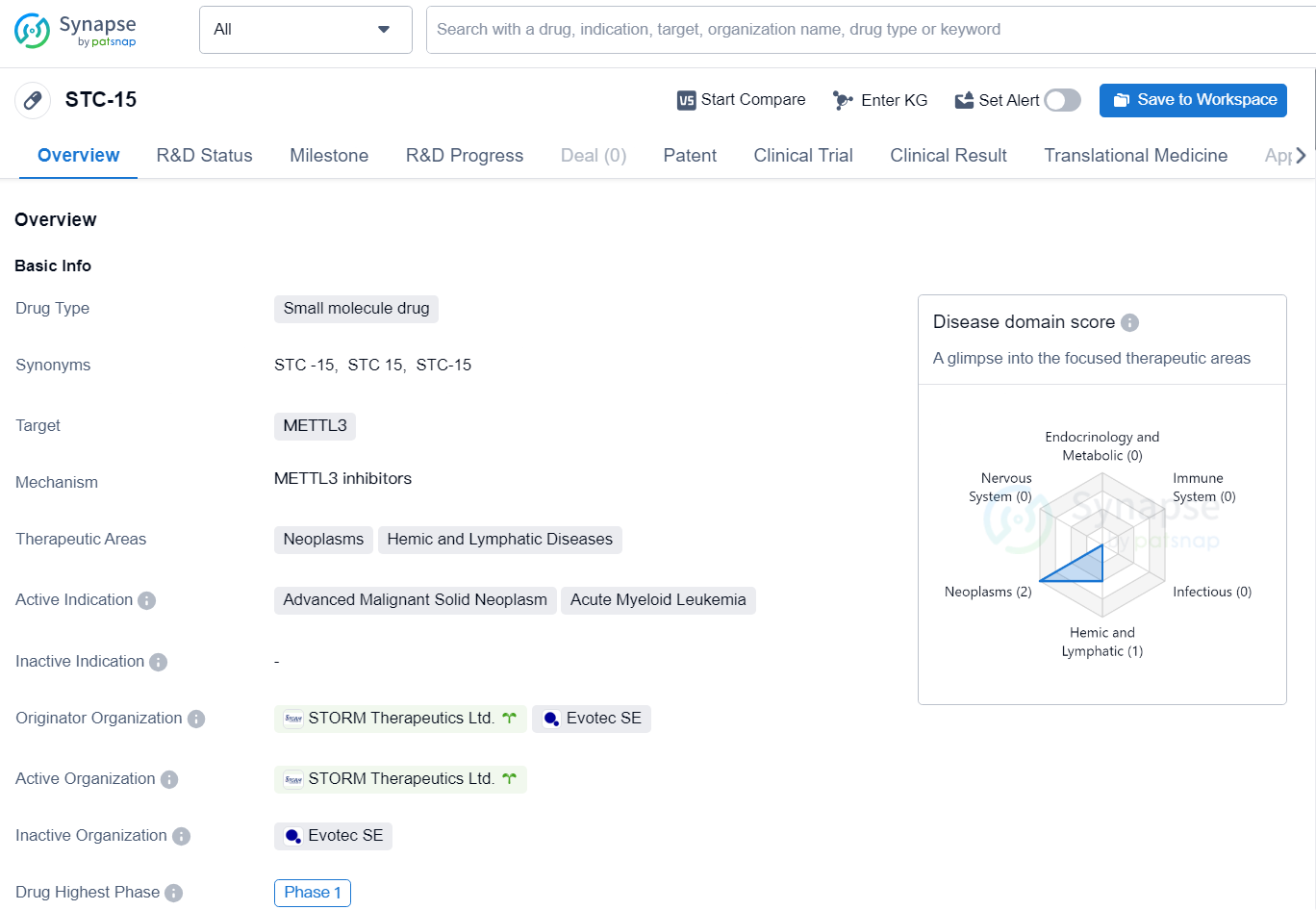
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The initial clinical trial Phase 1 involved 42 participants across five groups for dose escalation, ranging from 60mg to 200mg, and examined both daily and thrice weekly oral dosing schedules. The SITC data presentation follows the Company's participation at the 36th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics held in October 2024.
During the SITC session, a presentation titled "Phase 1 dose escalation and cohort expansion study assessing safety, PK, PD, and clinical activity of STC-15, a METTL3 inhibitor, in individuals with advanced cancers" revealed:
- Gene expression pathways linked to Interferon (IFN) signaling, virus response, and dsRNA binding were elevated in those who underwent prolonged STC-15 treatment.
- Tumor regressions were seen at all dosing levels, 60 mg to 200 mg, thrice weekly (TIW), achieving three sustained Partial Responses (PRs) at 60 mg, 100 mg, and 200 mg TIW across various tumor types.
- Emerging side effects from treatments, including target-related adverse events (such as reduced platelets, rash, itching), were generally mild, temporary, and effectively managed with supportive care, not hindering the treatment continuation.
- There was no maximum tolerated dose determined up to 200 mg TIW.
- Presence of M1 macrophages in the tumor microenvironment aligned with preclinical findings.
- Strong METTL3 target engagement was shown by rapid and sustained inhibition of m6A.
Kyriakos P. Papadopoulos, Co-Director of Clinical Research at START San Antonio, principal investigator for the STC-15 Phase 1 study, and lead author of the SITC presentation, stated: "The distinctive aspect of STC-15 is its capacity to activate the innate immune system while preserving tolerability and effectiveness. STC-15 targets METTL3, initiating a new immunologic mechanism that exhibits exceptional safety for an immuno-oncology drug. The initial efficacy and safety data in the context of advanced cancer support further clinical development in Phase 2 across diverse tumor types."
Jerry McMahon, CEO of STORM Therapeutics, remarked: "STC-15 is pioneering-first to target METTL3 and a RNA methyltransferase. We are delighted to convey promising signs of clinical activity and related upregulation of gene pathways involved with the METTL3 mechanism. We anticipate advancing our product to a Phase 2 study following these optimistic results."
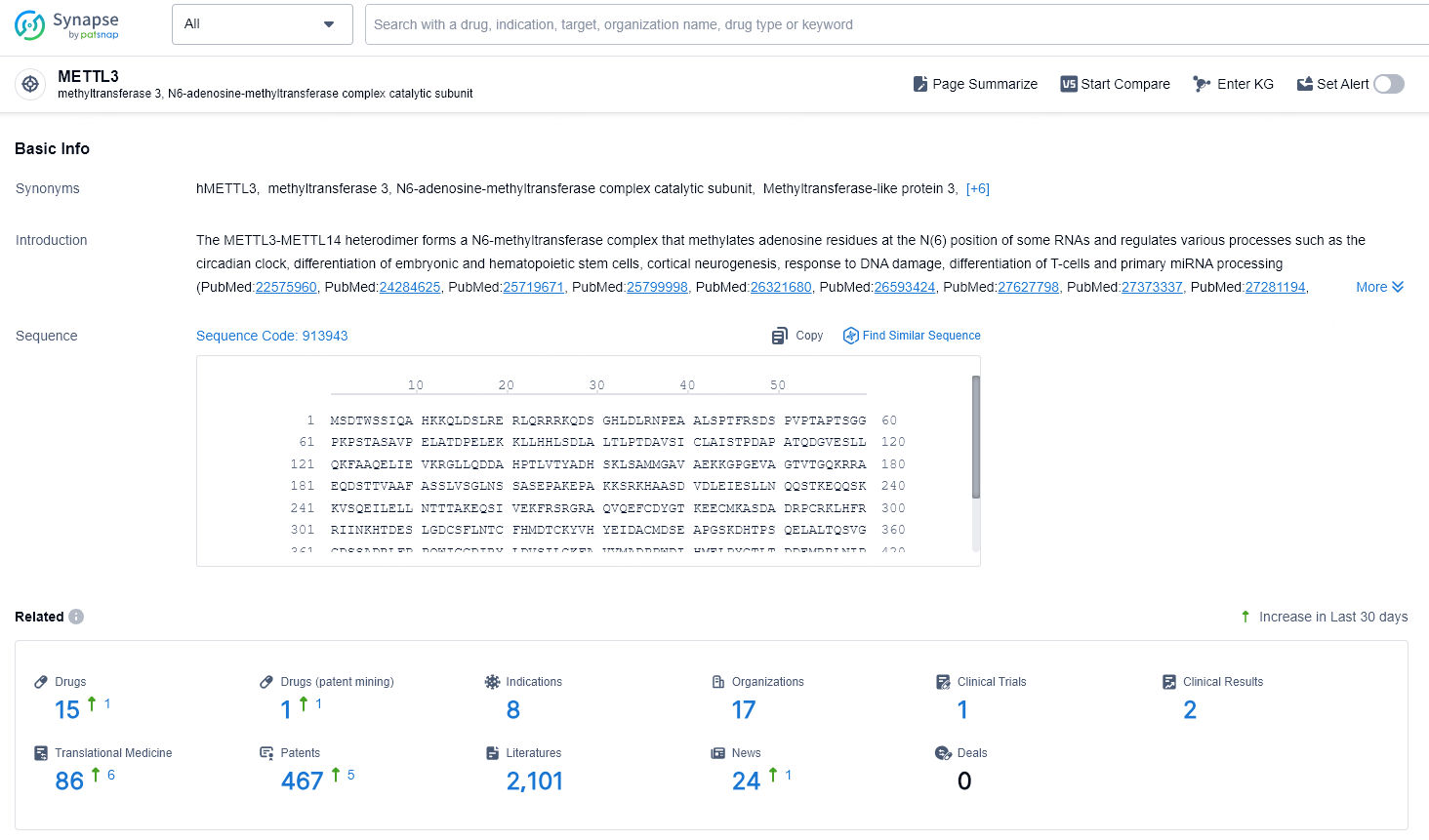
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 13, 2024, there are 15 investigational drugs for the METTL3 target, including 8 indications, 17 R&D institutions involved, with related clinical trial reaching 1, and as many as 467 patents.
STC-15 is a small molecule drug targeting METTL3, developed by STORM Therapeutics Ltd. and Evotec SE. This drug is primarily focused on the therapeutic areas of neoplasms and hemic and lymphatic diseases, with active indications for advanced malignant solid neoplasm and acute myeloid leukemi.