Marengo Unveils Promising Early Results for Invikafusp Alfa (STAR0602) at 2024 SITC Meeting
Marengo Therapeutics, Inc., a biotechnology firm in the clinical stage that is leading the way in innovative techniques for precise T cell activation, has revealed promising preliminary Phase 1 clinical results from its primary program, invikafusp alfa (STAR0602), during a late-breaking presentation at the 39th Annual Meeting of the Society for Immunotherapy of Cancer (SITC) held in Houston, Texas.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
This marks the initial public release of findings from the ongoing STARt-001 Phase 1/2 clinical trial (NCT05592626), which is assessing invikafusp alfa as a monotherapy for patients with advanced anti-PD-1 resistant or refractory solid tumors, specifically those enriched with biomarkers such as TMB-H, MSI-H/dMMR, or virally associated characteristics.
Data from the Phase 1 portion of the STARt-001 trial have revealed early evidence of anti-tumor efficacy, indicating potential clinical benefit in heavily pre-treated cancer patients resistant to anti-PD-1 therapy. Invikafusp alfa has also demonstrated a manageable safety profile that aligns with its innovative mechanism of action, enhancing its potential applicability in various high tumor mutational burden (TMB-H) cancers and virally linked malignancies.
“After completing Phase 1 and initiating the dose expansion segments in Phase 2 of STARt-001, Marengo is excited to present early clinical data that substantiate our unique selective dual T cell agonist platform," stated Zhen Su, M.D., MBA, Chief Executive Officer of Marengo Therapeutics. "The efficacy observed in Phase 1, particularly in cold tumors resistant to PD-1, such as colorectal cancer, represents a significant milestone, and we are eager to further investigate the potential of STAR0602 as a next-generation foundational immuno-oncology therapy for diverse tumor types.”
Key highlights from the Phase 1 results include:
- A significant and sustained in vivo expansion of TCRVβ6/Vβ10 T cells was noted across all six dose levels, achieving up to approximately 500% peak increase following treatment with invikafusp alfa.
- The Disease Control Rate (including partial response and stable disease) was reported in 50% of the 28 patients across all dose escalation cohorts, with 32% showing tumor reduction across six different tumor types.
- At the optimal biological dose range (0.08 mg/kg and 0.12 mg/kg), invikafusp alfa exhibited single-agent activity with a 63% Disease Control Rate; 50% of patients experienced tumor shrinkage, and a 25% overall response rate (ORR) was reported in TMB-H patients resistant to anti-PD-1 therapies.
- The safety profile was consistent with the mechanism of action related to T cell activation and expansion, without requiring pre-treatment with corticosteroids or tocilizumab. The most frequently observed treatment-related adverse events (TRAEs) were primarily transient grade 1 and 2 cytokine release syndrome (CRS) during the first and second infusions, with no occurrences of grade 4 adverse events or immune effector cell-associated neurotoxicity syndrome (ICANS).
- The recommended Phase 2 dose (RP2D) of 0.08 mg/kg was chosen for the Phase 2 expansion studies based on safety, pharmacokinetics/pharmacodynamics (PK/PD) data, and preliminary anti-tumor activity results.
"The initial human data indicate that this innovative approach to selectively activate and expand Vβ T cell subsets may offer potential benefits for patients with advanced solid tumors," remarked Dr. James L. Gulley, Co-Director of the Center for Immuno-Oncology and Clinical Director at the National Cancer Institute. "The distinct Vβ T cell biology observed in humans, along with the specific expansion of Vβ6/Vβ10 across various solid tumors and initial anti-tumor effectiveness, particularly in heavily pre-treated anti-PD-1 resistant colon cancer patients with high TMB, are promising indicators. The unique clinical profile supports further exploration of this innovative mechanism of action in the next phase of trials addressing significant unmet medical needs in anti-PD-1 resistant cancers."
In summary, the findings from the STARt-001 study highlight the potential of invikafusp alfa as a novel treatment option for patients with advanced, PD-1-resistant solid tumors. Marengo has started the Phase 2 dose expansion and plans to release initial results in the second half of 2025.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
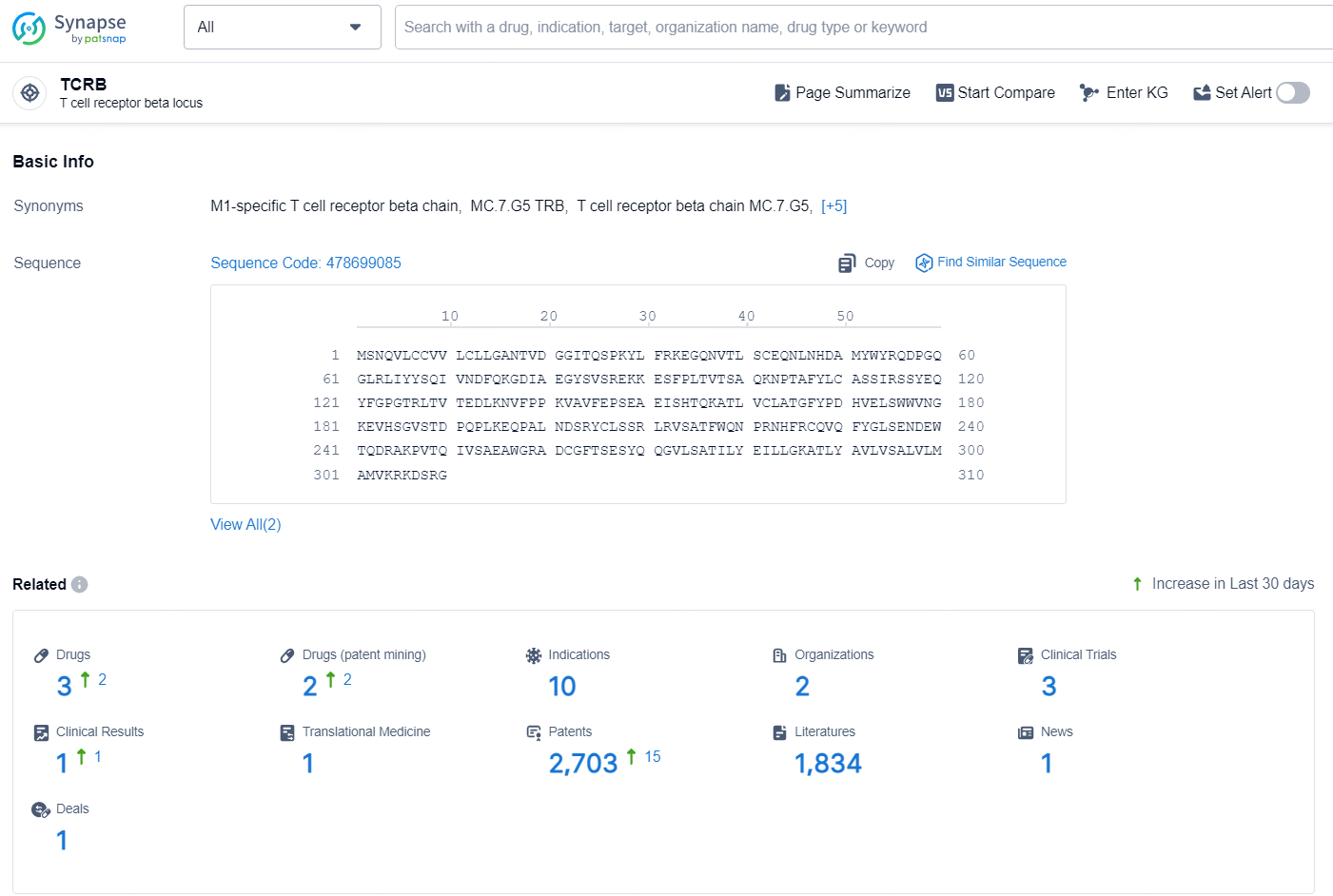
According to the data provided by the Synapse Database, As of November 13, 2024, there are 3 investigational drugs for the TCRB target, including 10 indication, 2 R&D institutions involved, with related clinical trials reaching 3, and as many as 2703 patents.
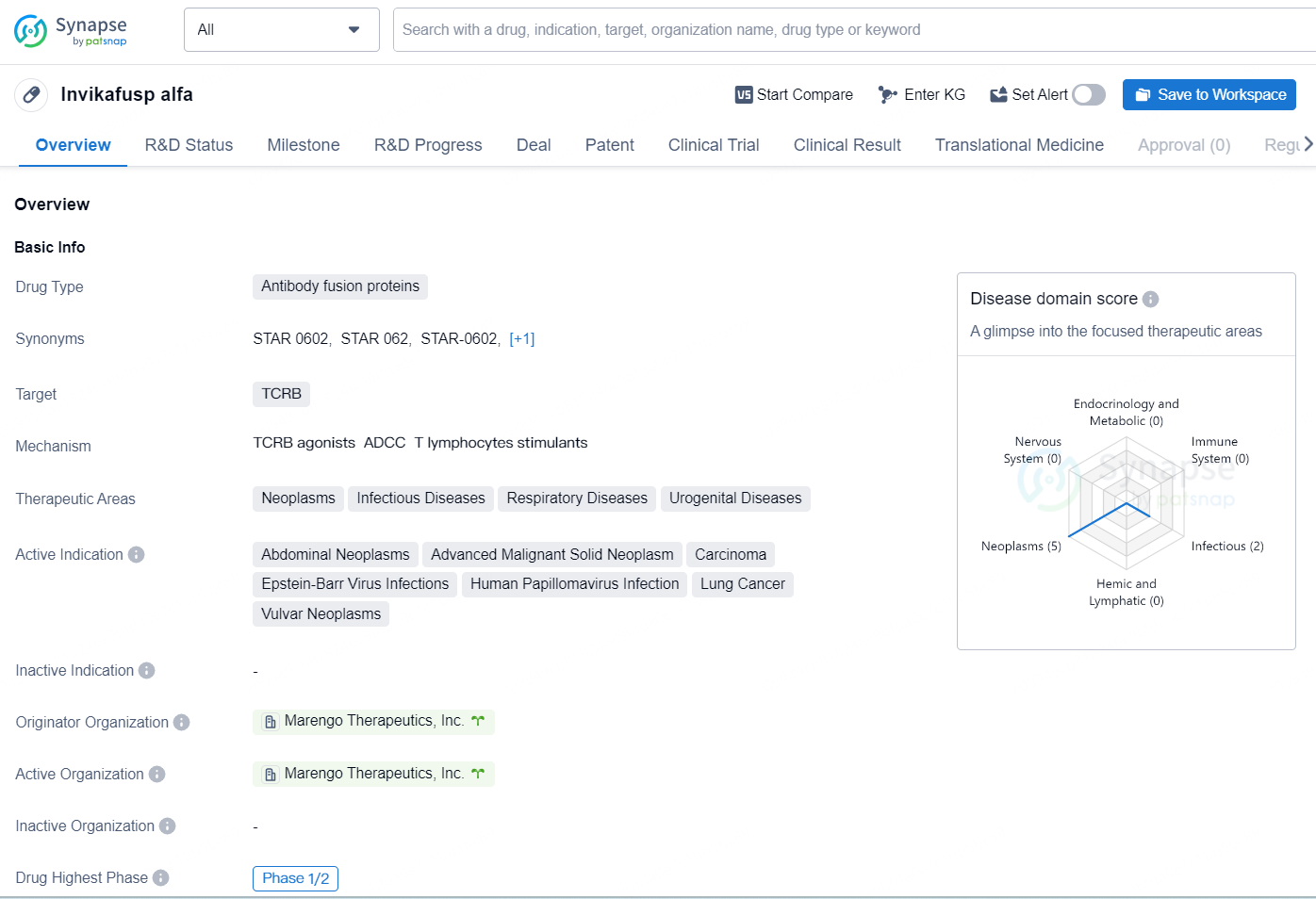
Invikafusp alfa is a drug classified as an antibody fusion protein, and it targets the TCRB. The therapeutic areas for this drug include neoplasms, infectious diseases, respiratory diseases, and urogenital diseases. The specific active indications for this drug are abdominal neoplasms, advanced malignant solid neoplasm, carcinoma, Epstein-Barr virus infections, human papillomavirus infection, lung cancer, and vulvar neoplasms.