Revolutionizing Drug Development: The Impact of AI on siRNA Design
The application of AI technology in new drug development spans several critical stages, from compound synthesis to drug repurposing. In the compound synthesis phase, AI optimizes synthesis routes by learning chemical reaction patterns. For crystal form prediction, AI uses computational simulations to forecast the optimal drug crystalline structure, enhancing drug performance. AI also evaluates pharmacological effects, identifying drug activity-related biomarkers from large data sets and predicting potential side effects. Additionally, AI technology enables drug repurposing by analyzing existing drug-disease relationships, accelerating the market entry of drugs and reducing development costs.
In developing new indications, AI leverages multi-omics data, such as genomics and transcriptomics, to identify disease-associated biological pathways, providing new targets for drug development. Moreover, AI demonstrates strong capabilities in safety and toxicity prediction by learning from historical data to anticipate potential safety issues in compounds, helping to eliminate unsuitable drug candidates early. AI is also used to optimize clinical trial design, analyzing patient data to identify the most suitable trial participants, thereby increasing trial success rates.
Particularly in siRNA drug development, AI’s application in siRNA screening and chemical modifications has brought revolutionary advancements to gene silencing research and drug development. Through machine learning models, AI identifies characteristics closely related to siRNA activity from extensive experimental data, predicting the potential effectiveness of untested siRNA sequences.
AI in siRNA Activity Prediction
AI has significantly improved precision in siRNA activity prediction. For instance, siRNA activity prediction, based on deep learning, integrates features like sequence context and thermodynamic properties, achieving more accurate predictions of siRNA inhibition efficacy than existing methods. This method enhances the precision of siRNA design, reducing trial-and-error costs and enabling faster screening of highly effective siRNA sequences.
siRNA Design Tools
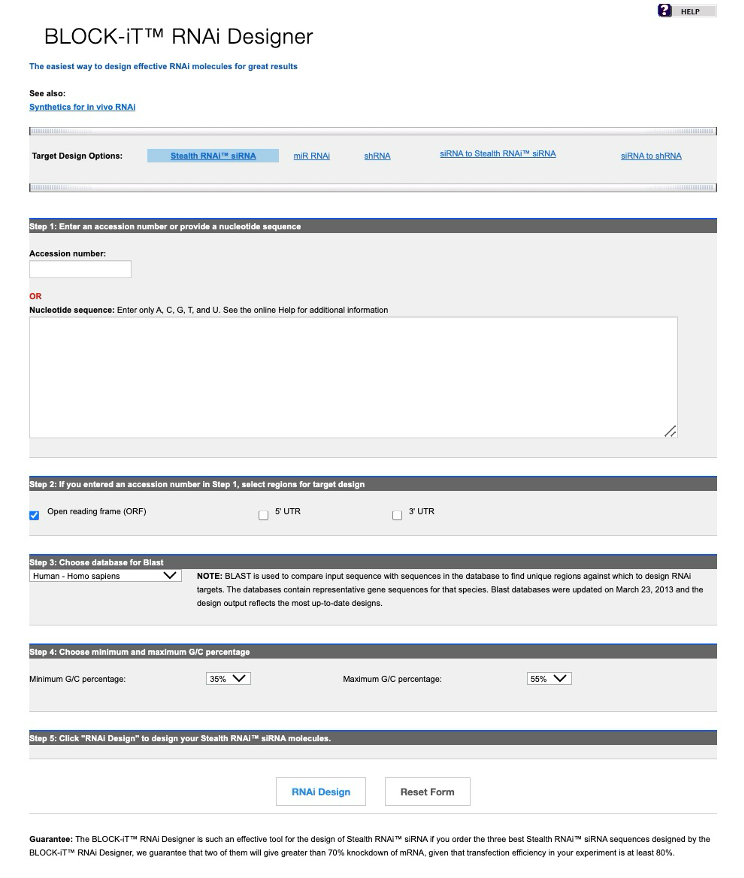
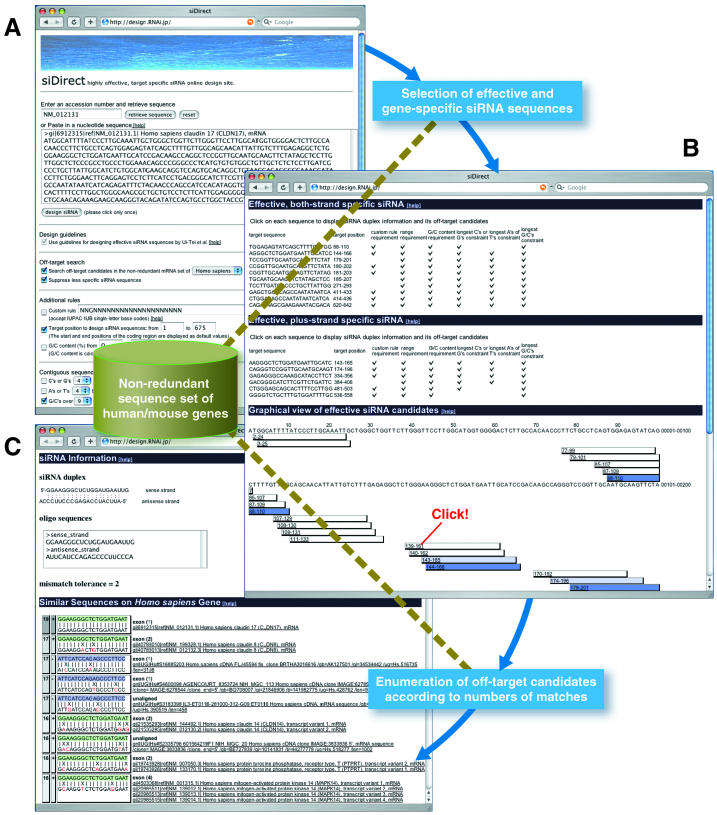
BLOCK-iT™ RNAi Designer, offered by Thermo Fisher Scientific, is an online design tool that assists researchers in designing siRNA based on specific gene sequences. In addition to standard siRNA design, it supports chemically modified siRNA options. Users input the target mRNA sequence, and BLOCK-iT™ RNAi Designer identifies likely effective siRNA sequences using its built-in algorithm, providing a detailed analysis report. The tool also supports designing Stealth RNAi™ siRNA, a chemically modified siRNA with enhanced stability and reduced immunogenicity, improving siRNA efficacy. Another commonly used tool is siDirect, which focuses on designing effective siRNA sequences. siDirect employs strict selection criteria, including avoiding single nucleotide polymorphisms (SNP) sites, non-open reading frame (ORF) segments, and unfavorable secondary structures, ensuring high specificity and low off-target effects for designed siRNA.
AI-Driven siRNA Chemical Modifications
AI technology can also play a significant role in siRNA chemical modification strategies. By analyzing siRNA sequences and chemical modification patterns, AI aids researchers in designing more effective siRNA drugs. For instance, one study applied machine learning models to classify effective chemically modified siRNA based solely on sequence and modification patterns, offering new insights into siRNA drug development.
Cm-siRPred is an algorithm designed to predict the efficiency of chemically modified small interfering RNAs (cm-siRNA) using a multi-view learning strategy. Chemical modifications in siRNA involve altering specific structures to enhance stability, reduce immune responses, or increase targeting specificity. Cm-siRPred achieves more accurate predictions by representing various properties of cm-siRNA through distinct “views.” This strategy considers the double-stranded sequence, chemical modifications, and physical-chemical properties of cm-siRNA, each providing unique insights. For the double-stranded sequence, the algorithm factors in base pairing and potential secondary structures that influence the siRNA’s ability to bind target mRNA, impacting its silencing efficiency. Integrating these variables, Cm-siRPred is a powerful tool in designing cm-siRNA with improved stability and specificity.
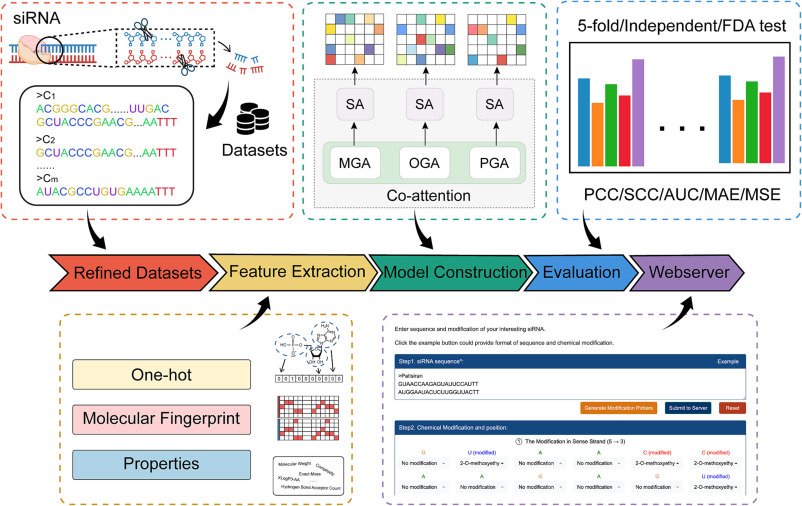
Additionally, Cm-siRPred assesses the influence of chemical modifications on cm-siRNA’s stability, immunogenicity, and cellular uptake. This combined information enables Cm-siRPred to evaluate the potential of a given cm-siRNA to silence target gene expression effectively. This algorithm significantly streamlines the design process, allowing researchers to pre-screen promising chemical modification strategies and enhance the efficiency and success rates of drug design.
Patsnap Bio’s Support for siRNA Drug Development
In siRNA drug development, selecting the correct target gene is a critical initial step. Patsnap Bio provides researchers with an extensive database of gene sequence data, enabling precise sequence searches. This resource allows researchers to quickly identify sequences related to the target gene and utilize advanced alignment tools to evaluate sequence similarity and differences, ensuring that designed siRNA specifically targets the intended gene.
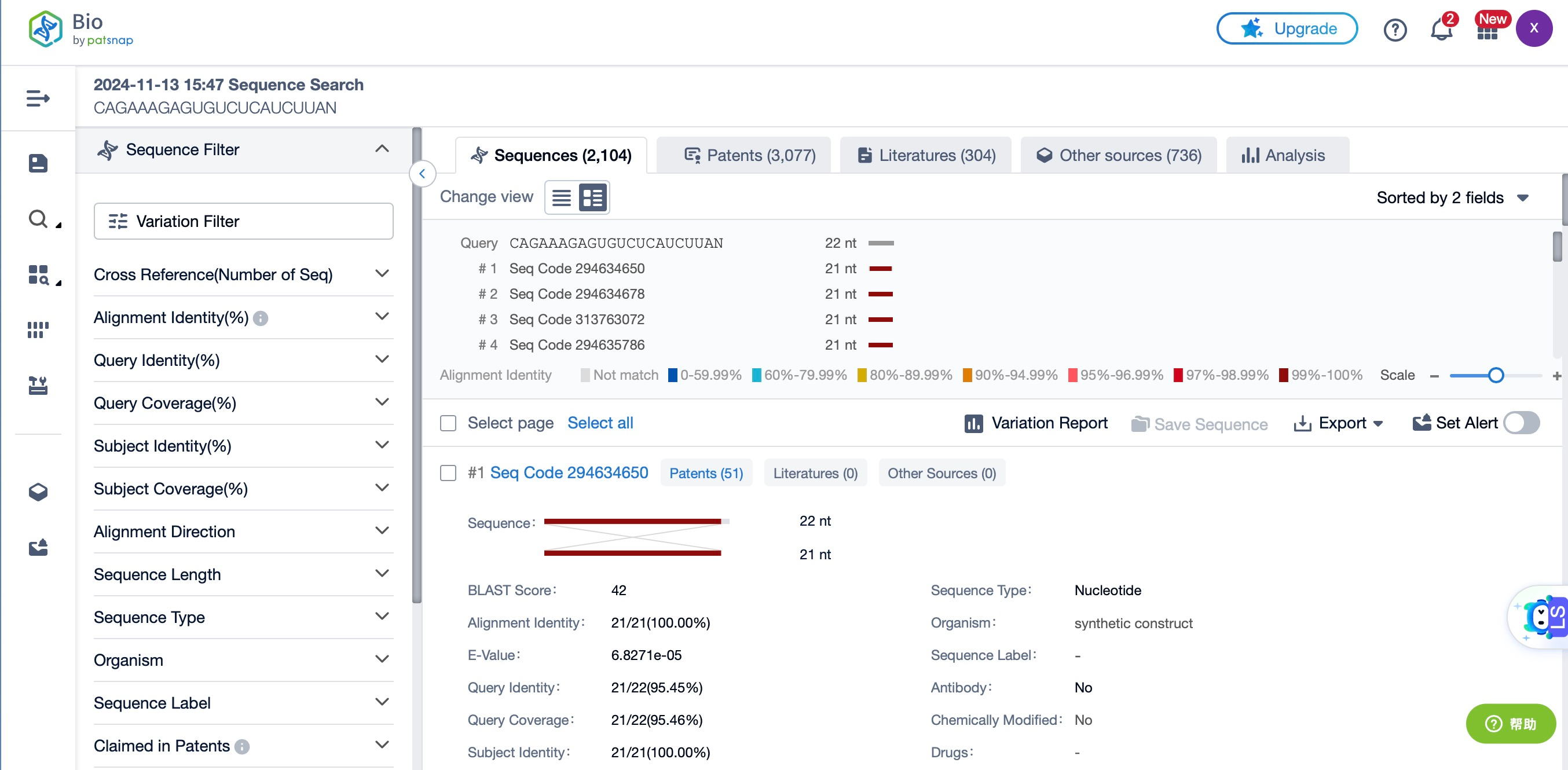
Patsnap Bio for Gene Function Annotation
Beyond gene sequences, Patsnap Bio offers comprehensive functional annotation data, covering gene functionality, biological processes, and cellular components. This information helps researchers understand the role of the target gene within the cell, which is essential for assessing the biological effects following siRNA intervention. Functional annotations also assist in avoiding sequences that might lead to non-specific silencing.
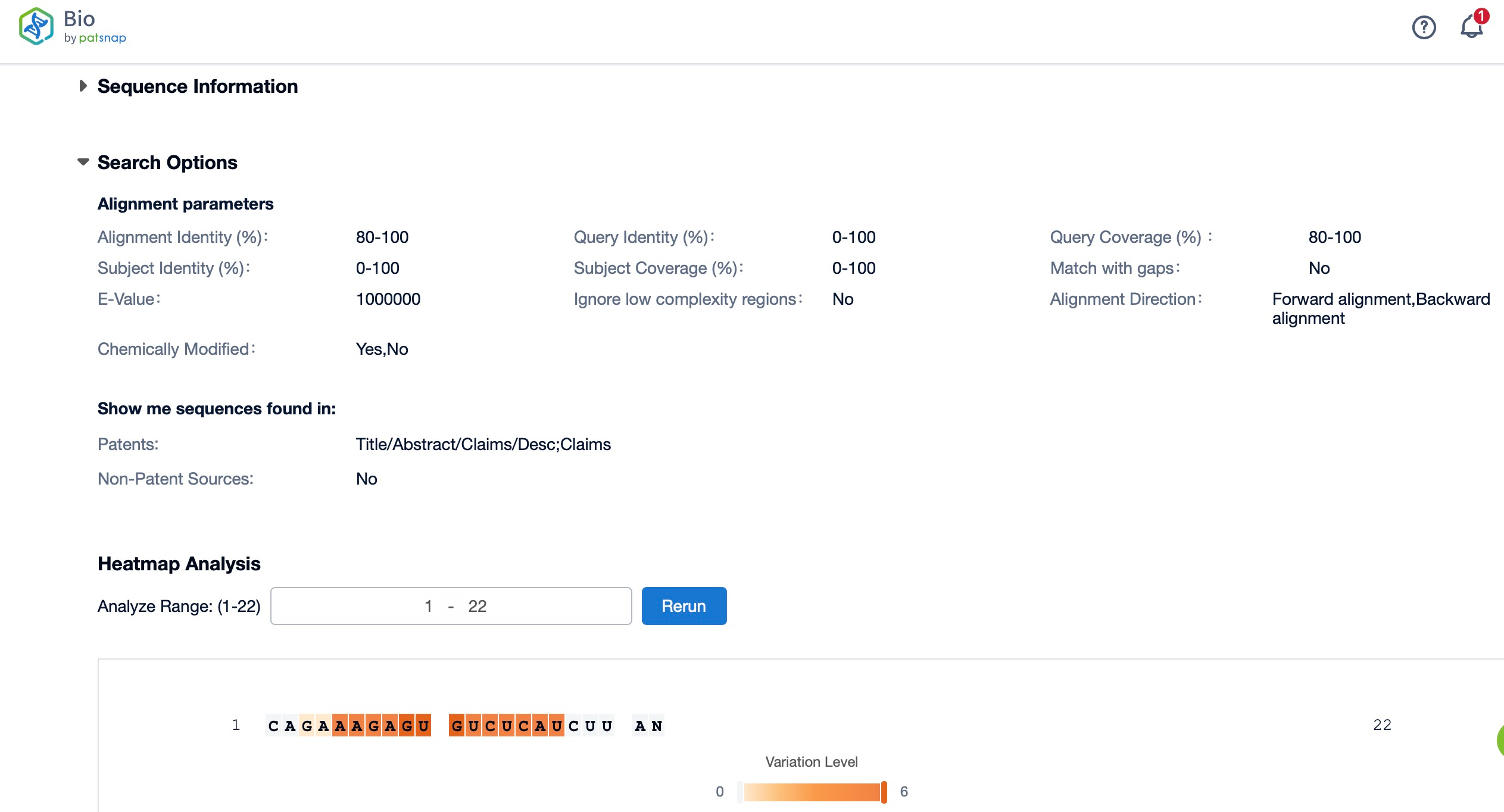
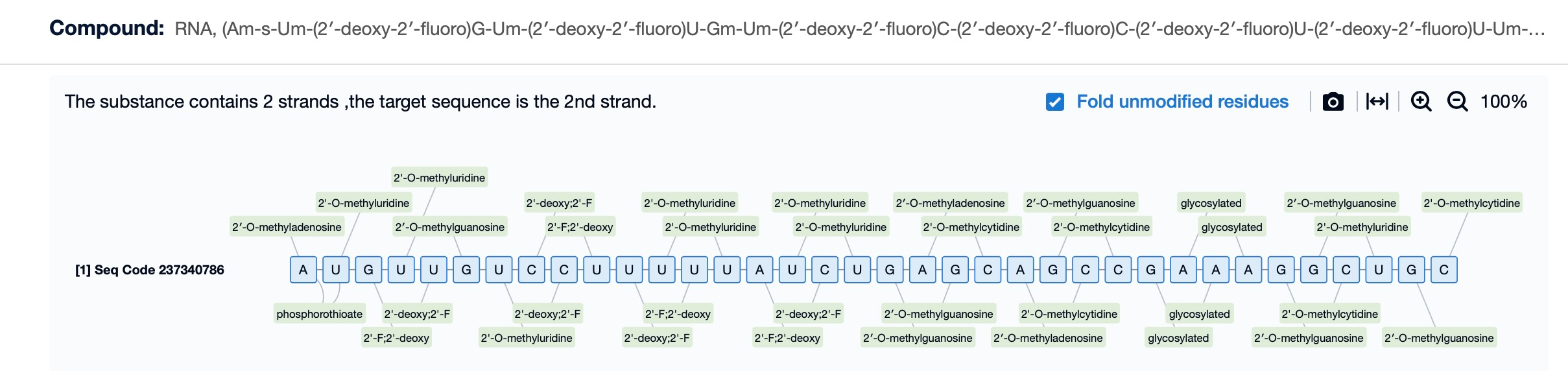
Patsnap Bio’s Insights for Chemical Modification Design
Chemical modification is a crucial step in enhancing siRNA stability and reducing off-target effects. Patsnap Bio provides foundational sequence data alongside research data on chemical modifications. Researchers can leverage this data to evaluate the impact of various chemical modifications on siRNA activity, optimizing the design for greater stability and specificity. By reviewing existing modification strategies, researchers can accelerate the development of more effective siRNA drugs.
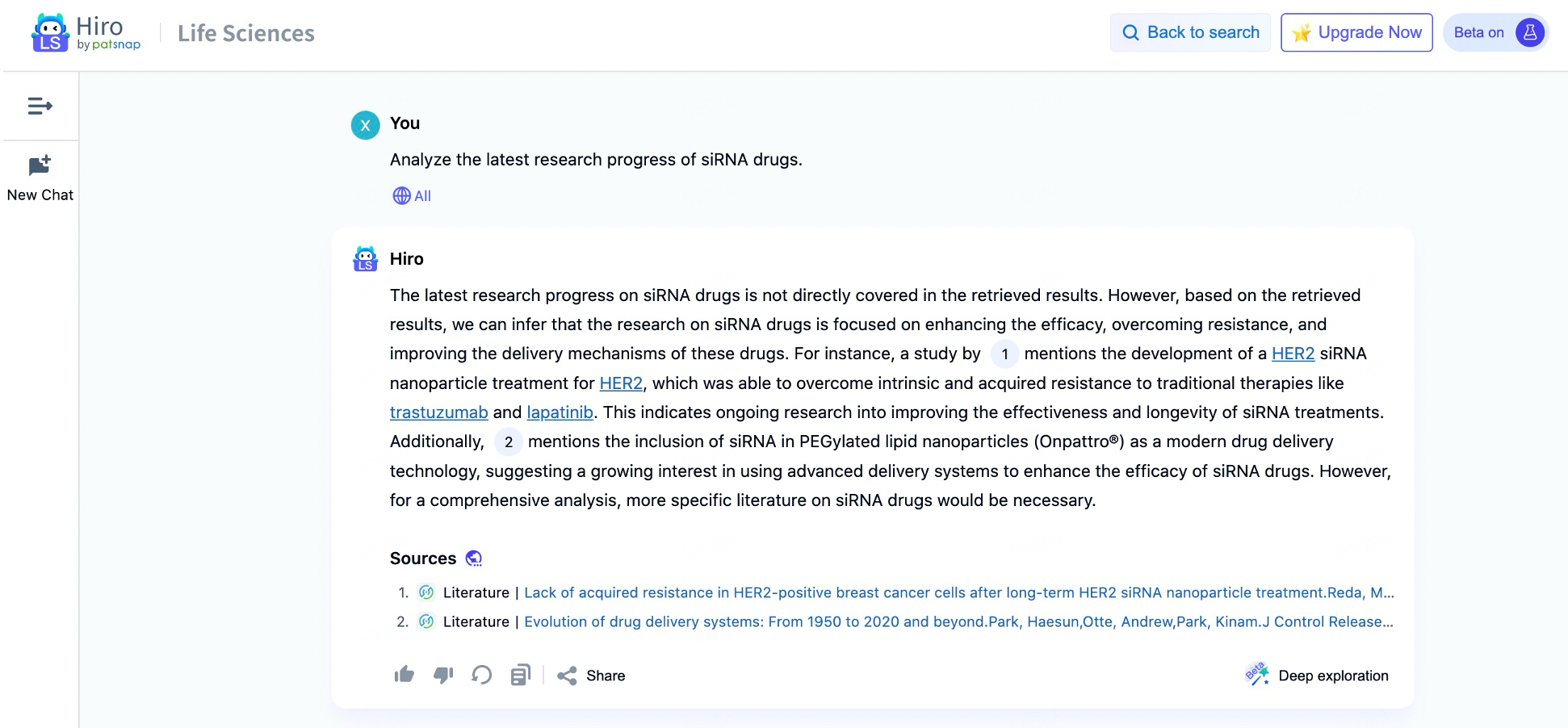
Hiro-LS: The AI Assistant for siRNA Drug Development
Hiro-LS is a large language model tailored to the biomedicine field, designed to provide expert-level information on biology, medicine, and pharmaceutical research. Hiro-LS enables researchers to quickly integrate and retrieve vast data relevant to siRNA drug development. Whether it involves recent research papers, clinical trial outcomes, or patent data, Hiro-LS’s intelligent retrieval system rapidly locates the latest advancements pertinent to siRNA drug development. This capability allows researchers to focus more on analyzing and applying information rather than spending extensive time gathering it. With interactive dialogue support, researchers can communicate directly with Hiro-LS to obtain instant technical support and clarify questions—whether related to siRNA design principles or specific experimental procedures. This human-AI interaction significantly streamlines information access, enhancing research efficiency.
In the siRNA drug development process, writing detailed research reports can be time-consuming. Hiro-LS has the capability to automatically generate preliminary technical reports. Based on provided keywords or research areas, it can organize and produce comprehensive reports that cover drug development progress, clinical trials, patent landscape, market size, and competitive positioning. This feature not only boosts productivity but also ensures the quality and thoroughness of the reports.
For an experience with the large-scale biopharmaceutical model Hiro-LS, please click here for a quick and free trial of its features!
Conclusion
The broad application of AI technology in new drug development not only accelerates the journey of drugs from laboratory to market but also provides novel solutions to traditional challenges in drug development. Especially in the design and optimization of siRNA drugs, AI significantly enhances stability and specificity through intelligent prediction and screening. As AI technology continues to evolve, its impact on drug development is expected to grow, contributing substantially to human health advancements.
With its extensive gene sequence information and detailed functional annotations, Patsnap Bio has become a powerful tool supporting siRNA drug development. By offering precise sequence search and alignment services and detailed chemical modification design references, Patsnap Bio greatly facilitates efficient siRNA drug design and optimization. As data collection and analysis technologies advance, Patsnap Bio will continue to provide strong support for siRNA drug research.
Hiro-LS opens a new chapter in siRNA drug development with its robust information integration, intelligent retrieval, and technical support capabilities. By automating report generation, providing data analysis support, and fostering knowledge sharing and collaboration, Hiro-LS not only enhances research efficiency but also drives innovation in siRNA drug development. As technology continues to develop and improve, Hiro-LS will play an increasingly important role in siRNA drug research, contributing to the progress of the pharmaceutical industry.
Reference
- 1.Naito, Y., Yamada, T., Ui-Tei, K., Morishita, S. & Saigo, K. siDirect: highly effective, target-specific siRNA design software for mammalian RNA interference. Nucleic Acids Res 32, W124-129 (2004). https://doi.org:10.1093/nar/gkh442
- 2.Liu, T. et al. Cm-siRPred: Predicting chemically modified siRNA efficiency based on multi-view learning strategy. Int J Biol Macromol 264, 130638 (2024). https://doi.org:10.1016/j.ijbiomac.2024.130638