FDA Approves Astellas' VYLOY™ (zolbetuximab-clzb) for Advanced Gastric and GEJ Cancers
Astellas Pharma Inc. has reported that the U.S. Food and Drug Administration (FDA) has given the green light to VYLOY™ (zolbetuximab-clzb). This treatment is indicated in conjunction with fluoropyrimidine- and platinum-based chemotherapy for adult patients suffering from locally advanced unresectable or metastatic human epidermal growth factor receptor 2 (HER2)-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma, characterized by claudin CLDN18.2 positivity as confirmed through an FDA-sanctioned test. VYLOY stands as the sole FDA-approved therapy in the United States targeting CLDN18.2.
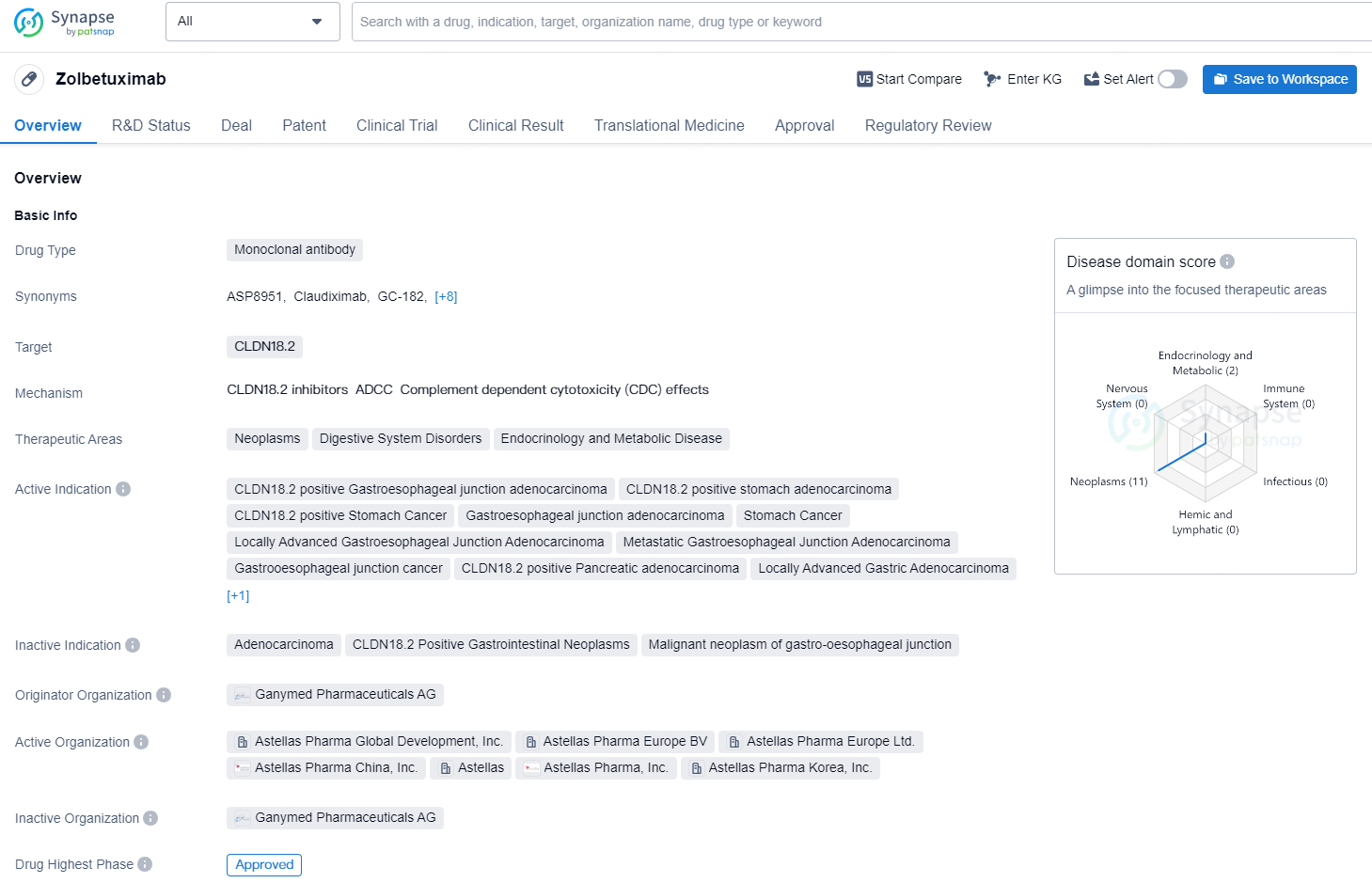
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In the SPOTLIGHT and GLOW clinical studies, around 38% of the patients evaluated had tumors that tested positive for CLDN18.2. CLDN18.2 positivity is established when at least 75% of tumor cells exhibit moderate to strong membranous staining for CLDN18, assessed using the VENTANA® CLDN18 (43-14A) RxDx Assay from Roche. Astellas has partnered with Roche to develop a newly sanctioned immunohistochemistry (IHC) companion diagnostic (CDx) test that helps identify patients who might qualify for VYLOY.
Moitreyee Chatterjee-Kishore, Ph.D., M.B.A., Senior Vice President and Head of Immuno-Oncology Development at Astellas, remarked, “The approval of VYLOY as the first and sole targeted treatment for CLDN18.2-positive patients in the U.S. embodies our unwavering commitment to scientific advancement for severe diseases such as gastric and gastroesophageal junction (GEJ) cancers, which are frequently diagnosed at advanced stages. This milestone is attributed to years of focused research and development aimed at targeting a novel biomarker, and we are thankful to the patients, researchers, and Astellas team members who have contributed to this significant progress for patients.”
Samuel J. Klempner, M.D., Associate Professor at Harvard Medical School and Medical Oncologist at Massachusetts General Hospital in Boston, stated, “Despite progress in first-line treatments for locally advanced unresectable and metastatic gastric and GEJ cancers in recent years, there remains a significant unmet need for our patients. The approval of VYLOY, derived from the pivotal Phase 3 SPOTLIGHT and GLOW trials, introduces a new biomarker and a novel treatment option for patients with CLDN18.2 positive tumors, aiding those involved in critical treatment decisions.”
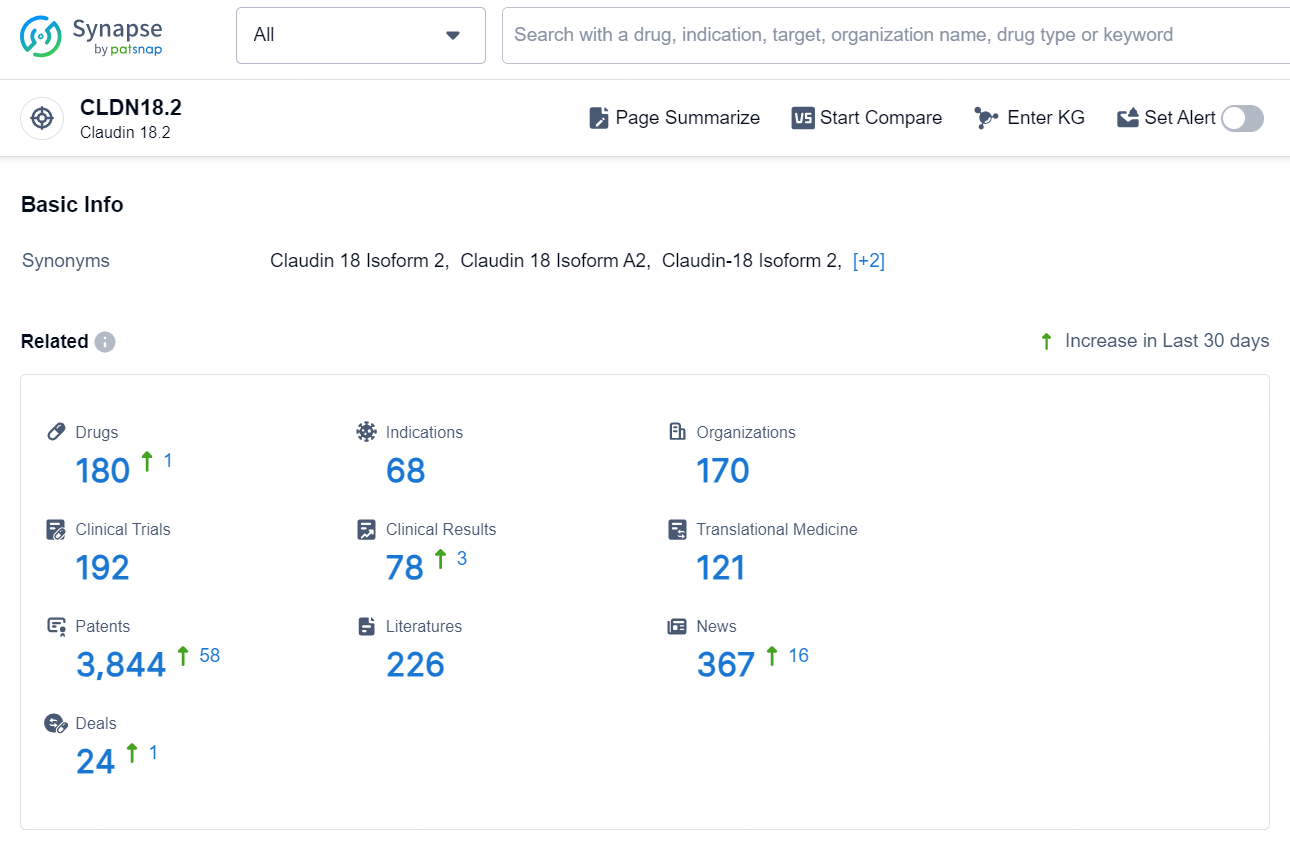
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 23, 2024, there are 180 investigational drug for the CLDN18.2 target, including 68 indications, 170 R&D institutions involved, with related clinical trials reaching 192, and as many as 3844 patents.
Zolbetuximab is a monoclonal antibody drug that targets CLDN18.2 and is utilized in the treatment of various neoplastic, digestive system disorders, endocrinology, and metabolic diseases. The drug is indicated for use in CLDN18.2 positive Gastroesophageal junction adenocarcinoma, stomach adenocarcinoma, stomach cancer, locally advanced Gastroesophageal Junction Adenocarcinoma, metastatic Gastroesophageal Junction Adenocarcinoma, pancreatic adenocarcinoma, locally advanced gastric adenocarcinoma, and pancreatic adenocarcinoma metastatic. The originator organization for Zolbetuximab is Ganymed Pharmaceuticals AG, and it has obtained the highest phase of approval globally, with a first approval date of March 2024 in Japan. The drug has been granted priority review and orphan drug status, and it has also reached the NDA/BLA phase in China.