FDA Approves CellVax's FK-PC101 IND Submission for Personalized Cancer Immunotherapy
Theragent Inc., an integrated Contract Development and Manufacturing Organization (CDMO) with a specialization in pioneering cell-based treatment innovations, has declared that its partner client, CellVax Therapeutics Inc., has been granted approval for its Investigational New Drug (IND) submission from the U.S. Food and Drug Administration (FDA) pertaining to FK-PC101. The process of enlisting participants for the upcoming Phase II trial, which is designed as a randomized study, is set to commence in March 2024. The initial phase of administering treatments to patients is anticipated to take place in the second quarter of 2024.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.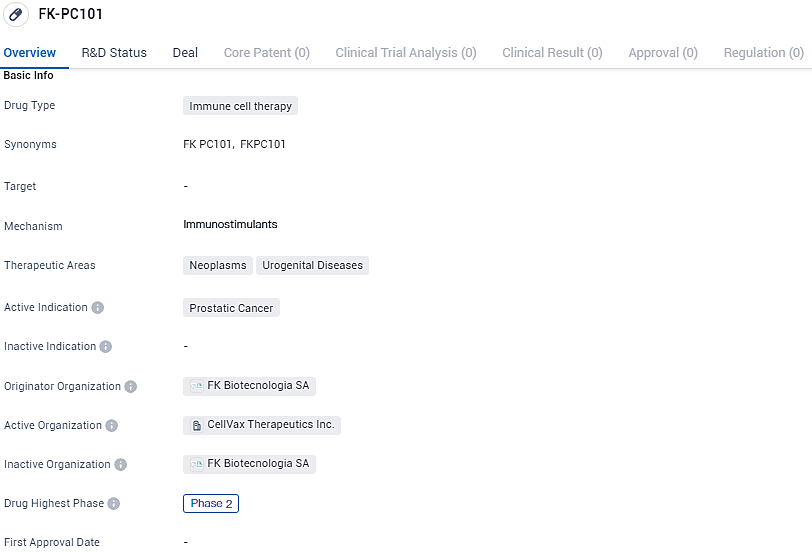
CellVax has developed a pioneering treatment for prostate cancer, known as FK-PC101. This cutting-edge approach focuses on patients with a heightened risk of the disease returning post-prostatectomy. Using the patient's own surgically removed tumor cells, the treatment involves a lab-based alteration of these cells to present the MHC Class II molecules on their surfaces. Post-modification, the cells are subjected to radiation to stop their proliferation, and subsequently utilized in a tailored immunotherapy regimen.
The head of CellVax, Fernando Kreutz, expressed his enthusiasm about the FDA approving their Investigational New Drug application for FK-PC101. Kreutz acknowledged the intense efforts during the application phases, in association with their trusted collaborator, Theragent. This collaboration has incorporated extensive experience in process improvement, navigating regulatory requirements, and maintaining CellVax's fundamental principles. Kreutz noted this achievement aligns with the company's 20+ years of continual efforts from the inception to the clinical phase, aiming to bring forth an innovative therapeutic option for patients profoundly in need of treatment.
The clinical study named CELLVX-230 involves a non-blinded, multicenter configuration and will involve male participants who've undergone a radical prostatectomy and are at significant risk. The clinical trial is set to launch through collaboration with the Society of Urologic Oncology Clinical Trials Consortium, overseen by Dr. Scott Eggener as the lead investigator. The enrollment for the study is anticipated to commence in March 2024, targeting a total of 230 volunteers from 21 carefully chosen locations throughout the United States.
Theragent has pledged to oversee the complete manufacturing cycle, including the final release and management of clinical substances. Their current facilities in Arcadia, California, are purposefully designed to adhere to CGMP standards for cell therapy production. Dr. Yun Yen, Theragent's president, stated their excitement to be working alongside CellVax on this groundbreaking clinical venture, underscoring Theragent's mission to deliver transformative treatments to those who desperately need them.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this indications in just a click!
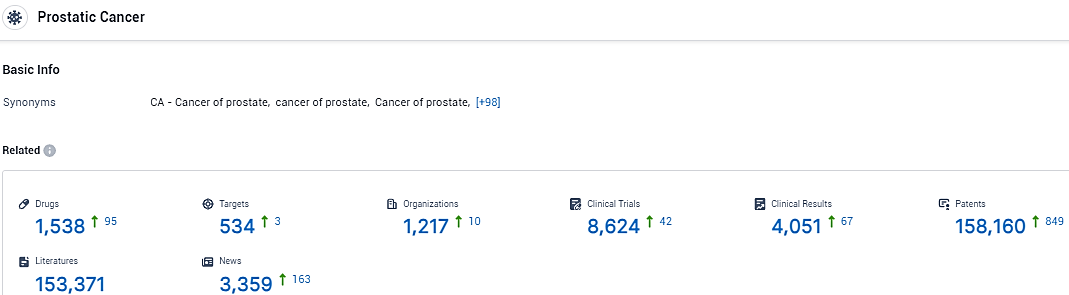 According to the data provided by the Synapse Database, As of February 6, 2024, there are 1538 investigational drugs for the prostate cancer, including 534 targets, 1217 R&D institutions involved, with related clinical trials reaching 8624, and as many as 158160 patents.
According to the data provided by the Synapse Database, As of February 6, 2024, there are 1538 investigational drugs for the prostate cancer, including 534 targets, 1217 R&D institutions involved, with related clinical trials reaching 8624, and as many as 158160 patents.
FK-PC101 represents an exciting advancement in the field of biomedicine, specifically in the treatment of prostatic cancer. As an immune cell therapy drug, it has the potential to offer a targeted and personalized treatment option for patients with this type of cancer. With its current status in Phase 2, further research and development will be conducted to determine the drug's safety and efficacy, bringing it closer to potential approval and availability for patients in need.




