FDA Approves Genentech's Itovebi for Specific Advanced Breast Cancer
Genentech, part of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), has announced that the U.S. Food and Drug Administration (FDA) has granted approval for Itovebi™ (inavolisib). This medication is indicated for use in combination with palbociclib (Ibrance®) and fulvestrant to treat adult patients suffering from endocrine-resistant, PIK3CA-mutated, hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer. This approval follows the detection of these conditions via an FDA-approved diagnostic test after the patient has experienced a recurrence post-completion of adjuvant endocrine therapy. The PIK3CA mutation occurs in roughly 40% of HR-positive metastatic breast cancer cases.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The PI3K signaling pathway is crucial in the development of various diseases and poses significant challenges for targeted therapies," stated Komal Jhaveri, M.D., who heads the endocrine therapy research and serves as clinical director of early drug development at Memorial Sloan Kettering Cancer Center, and is among the lead researchers of the INAVO120 trial. "The regimen based on Itovebi has demonstrated more than a twofold increase in progression-free survival while maintaining an acceptable safety and tolerability profile, establishing a new benchmark in the management of PIK3CA-mutated breast cancers."
“With the endorsement of this Itovebi-based treatment, we uphold our commitment to cancer therapeutic innovations by providing a vital new first-line alternative for patients with HR-positive breast cancer harboring a PIK3CA mutation,” remarked Levi Garraway, M.D., Ph.D., Genentech’s chief medical officer and leader of Global Product Development. “Though PIK3CA mutations are highly prevalent within this patient population, available treatment options have been sparse, which underscores the importance of today's approval.”
This endorsement is grounded in the findings from the critical Phase III INAVO120 trial, which indicated that the Itovebi regimen decreased the likelihood of disease progression or mortality by 57% when compared to palbociclib and fulvestrant alone (15.0 months vs. 7.3 months; hazard ratio [HR]=0.43, 95% CI: 0.32-0.59, p<0.0001) in a first-line treatment context, showing a statistically significant and meaningful clinical advantage. Data on overall survival (OS) were still premature at the time of the primary analysis, but a favorable trend was evident (stratified HR=0.64, 95% CI: 0.43-0.97, p=0.0338 [boundary of 0.0098]). Follow-up for OS continues for subsequent evaluations.
“We are exhilarated by the Itovebi regimen's approval, representing a significant advancement for patients with advanced breast cancer possessing a PIK3CA mutation,” commented Jean Sachs, CEO of Living Beyond Breast Cancer. “It remains essential that all patients have access to timely, comprehensive biomarker testing, enabling them to understand better the most advantageous treatment options for their specific tumor types.”
The Itovebi regimen received FDA Priority Review and Breakthrough Therapy Designation in May 2024 due to the INAVO120 trial findings. Results from INAVO120 are also being utilized for applications to other international health regulatory bodies, including the European Medicines Agency. Itovebi is expected to be accessible in the U.S. within the next few weeks. Early and comprehensive biomarker testing using FDA-approved tests, such as Foundation Medicine’s FoundationOne®Liquid CDx, can aid in identifying individuals with HR-positive, HER2-negative breast cancer and PIK3CA mutations.
Currently, Itovebi is being evaluated in various combinations through three company-sponsored Phase III clinical trials (INAVO120, INAVO121, INAVO122) targeting PIK3CA-mutated locally advanced or metastatic breast cancer. We are continuously exploring possibilities to broaden our clinical development efforts to meet the unmet needs of patients across different tumor types in oncology.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
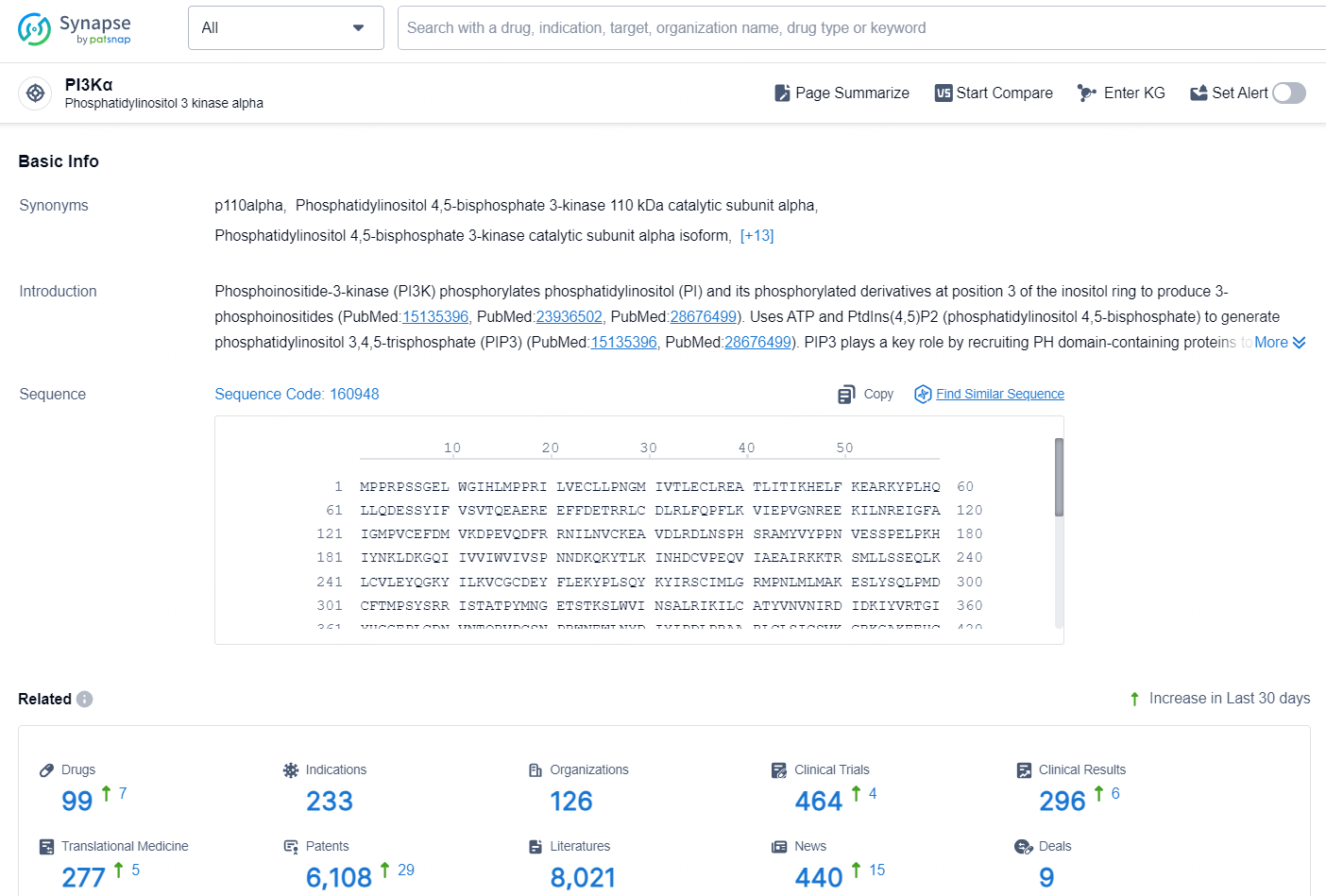
According to the data provided by the Synapse Database, As of October 15, 2024, there are 99 investigational drugs for the PI3Kα target, including 233 indications, 126 R&D institutions involved, with related clinical trials reaching 464, and as many as 6108 patents.
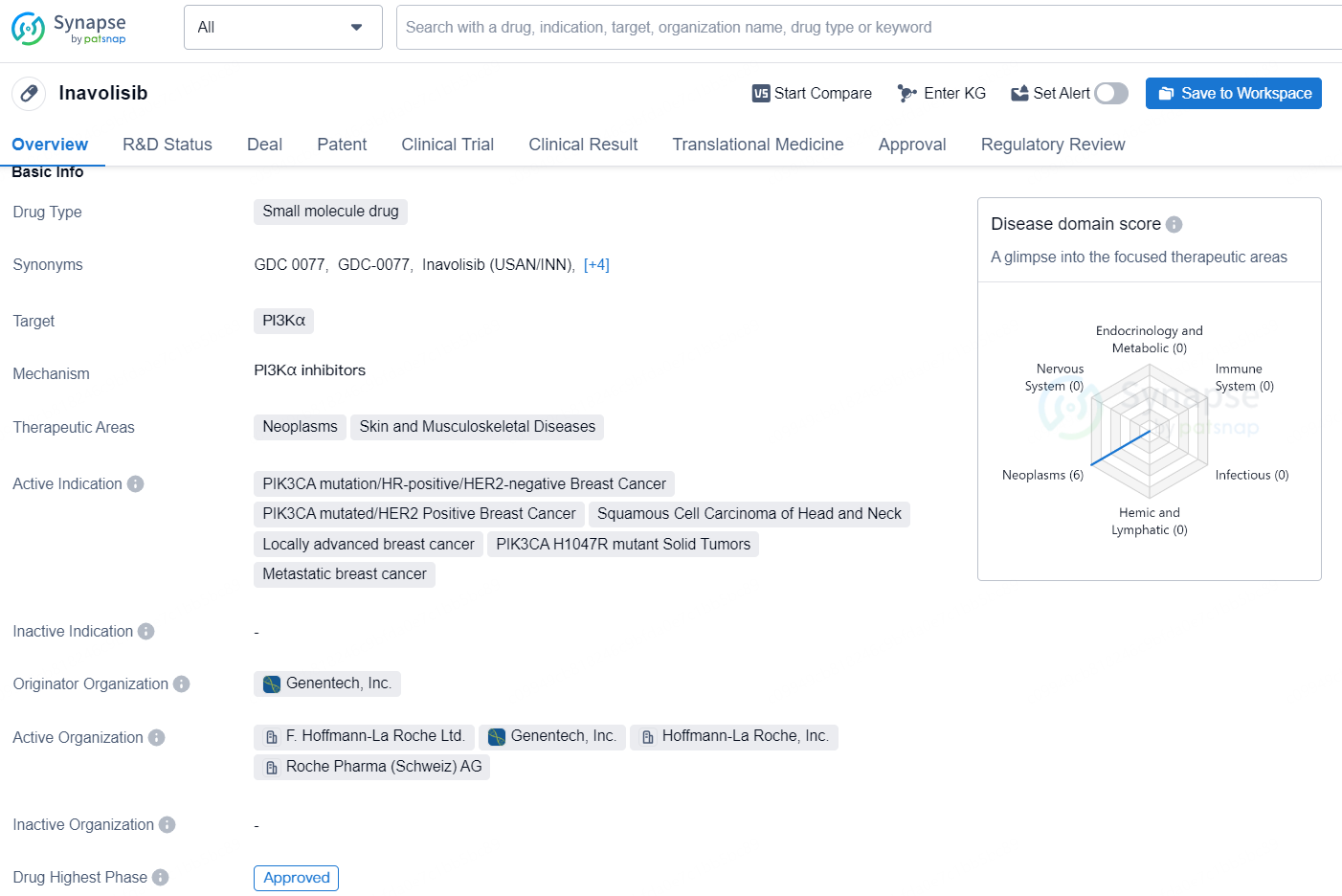
Inavolisib is a small molecule drug that targets PI3Kα and is indicated for various neoplasms, skin, and musculoskeletal diseases. The active indications for Inavolisib include PIK3CA mutation/HR-positive/HER2-negative Breast Cancer, PIK3CA mutated/HER2 Positive Breast Cancer, Squamous Cell Carcinoma of Head and Neck, Locally advanced breast cancer, PIK3CA H1047R mutant Solid Tumors, and Metastatic breast cancer. The originator organization of Inavolisib is Genentech, Inc.