FDA Approves IND Submission for Atara's ATA3219, an Off-the-Shelf CAR T Therapy for Lupus Nephritis
Atara Biotherapeutics, Inc., has disclosed that the FDA has granted approval to proceed with an IND (Investigational New Drug) for ATA3219. This investigational therapy is an allogeneic anti-CD19 CAR T-cell therapy designed specifically to address the complications of systemic lupus erythematosus that affects the kidneys.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Building on our rich clinical history with over 600 patients treated in fields of oncology and autoimmunity using our proprietary allogeneic T-cell platform, we are thrilled to advance into clinical assessments of our unique allogeneic CAR T-cell strategy. Our intention is to address the critical need in lupus nephritis by launching our Phase 1 study," announced Pascal Touchon, CEO and President at Atara.
Pascal Touchon continued, "We anticipate offering a pioneering and accessible one-size-fits-all cell therapy solution for individuals suffering from serious autoimmune conditions, potentially alleviating the challenges linked with autologous CAR T treatments, such as high costs and procedural delays."
This multi-site, Phase 1 trial is an open-label, single-group, dose-exploration study set to appraise the tolerability and initial biological response of ATA3219 in participants with lupus nephritis (LN). Participants will undergo a lymphodepleting pre-treatment, then receive an infusion of ATA3219 at varying doses of 40, 80, or 160 million CAR+ T cells.
The one-off infusion of ATA3219 is crafted to be monitored for both safety and response. Plans are in place for each dosage cohort to consist of 3 to 6 subjects, with the inaugural participant enrollment anticipated in the latter part of 2024.
The Investigational New Drug (IND) application for ATA3219 incorporated lab-generated evidence of the specificity of the CAR T-cells to the CD19 antigen and their ability to engage B cells derived from systemic lupus erythematosus (SLE) patients. The findings revealed that ATA3219 has a pronounced ability to eliminate CD19 targeted B cells when compared to standard options.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
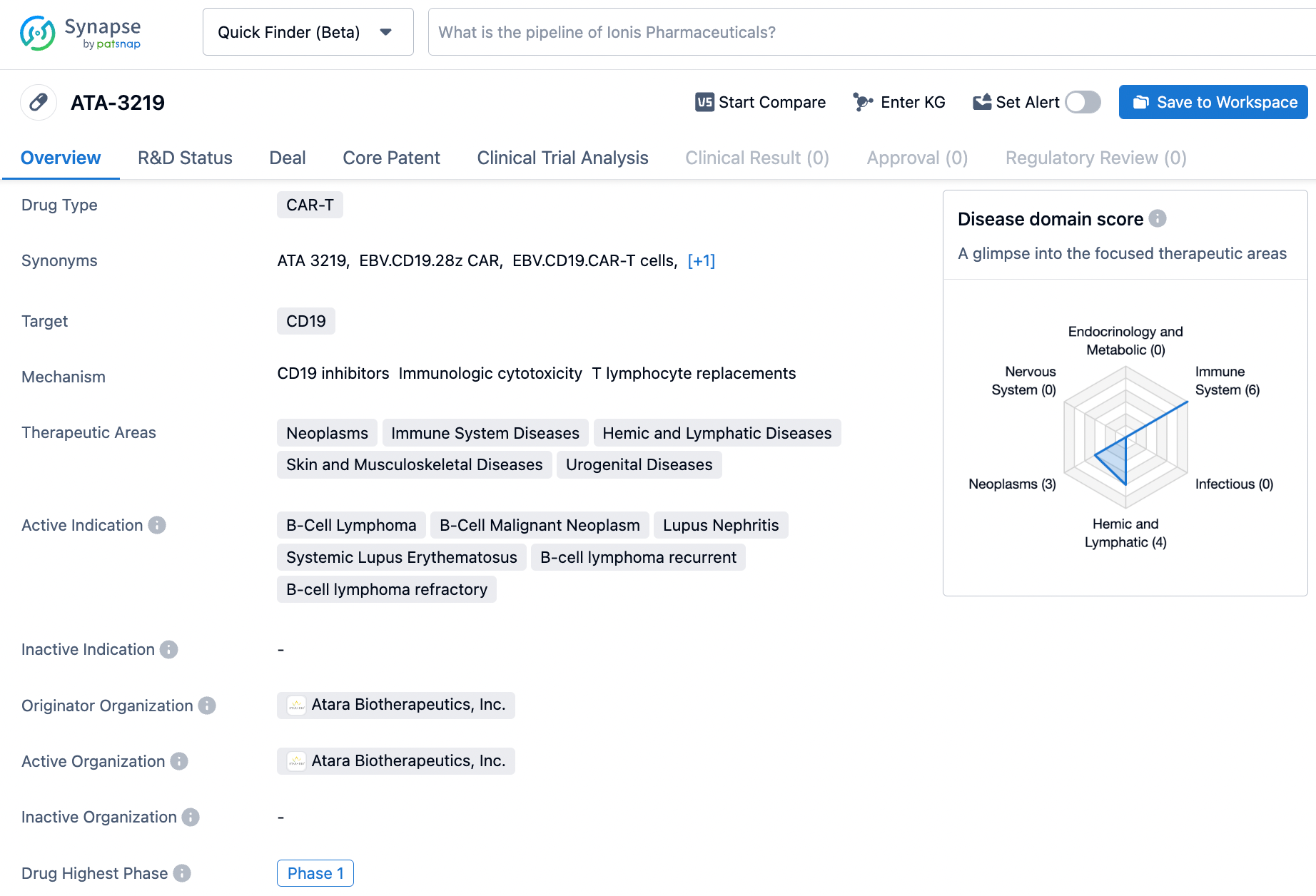
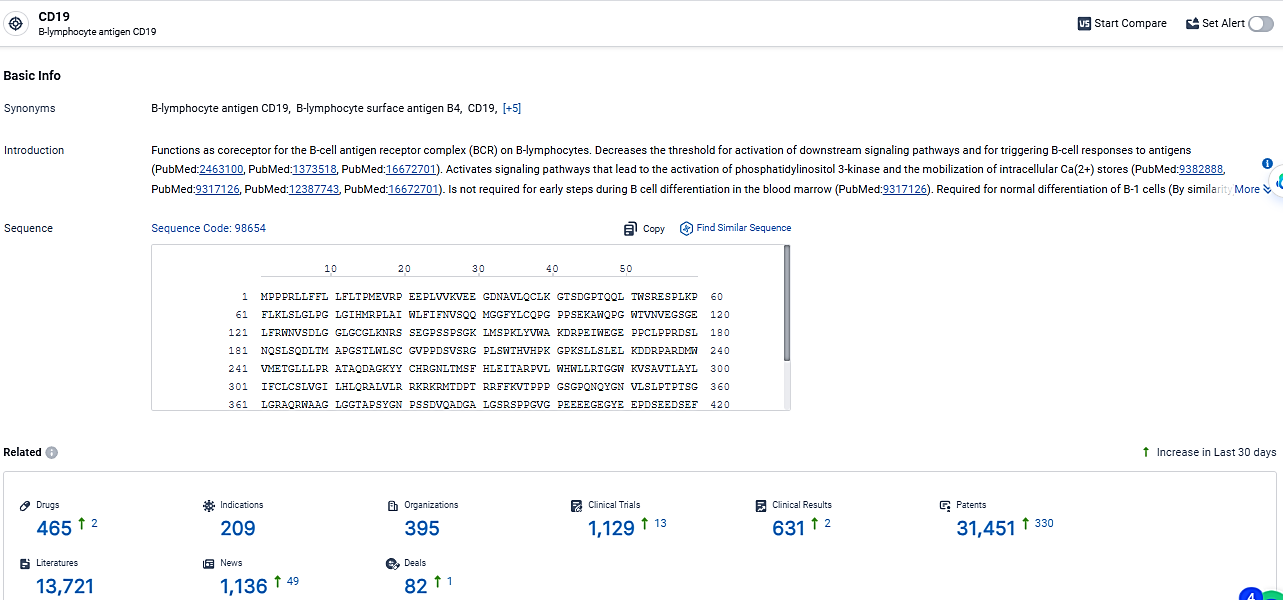
According to the data provided by the Synapse Database, As of March 5, 2024, there are 465 investigational drugs for the CD19 target, including 209 indications,395 R&D institutions involved, with related clinical trials reaching 1129, and as many as 31451 patents.
ATA-3219 targets CD19, a protein found on B-cells. It has potential applications in various therapeutic areas, including neoplasms, immune system diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, and urogenital diseases. The drug is currently in Phase 1 of development, indicating that it is being tested in humans to assess its safety and dosage.