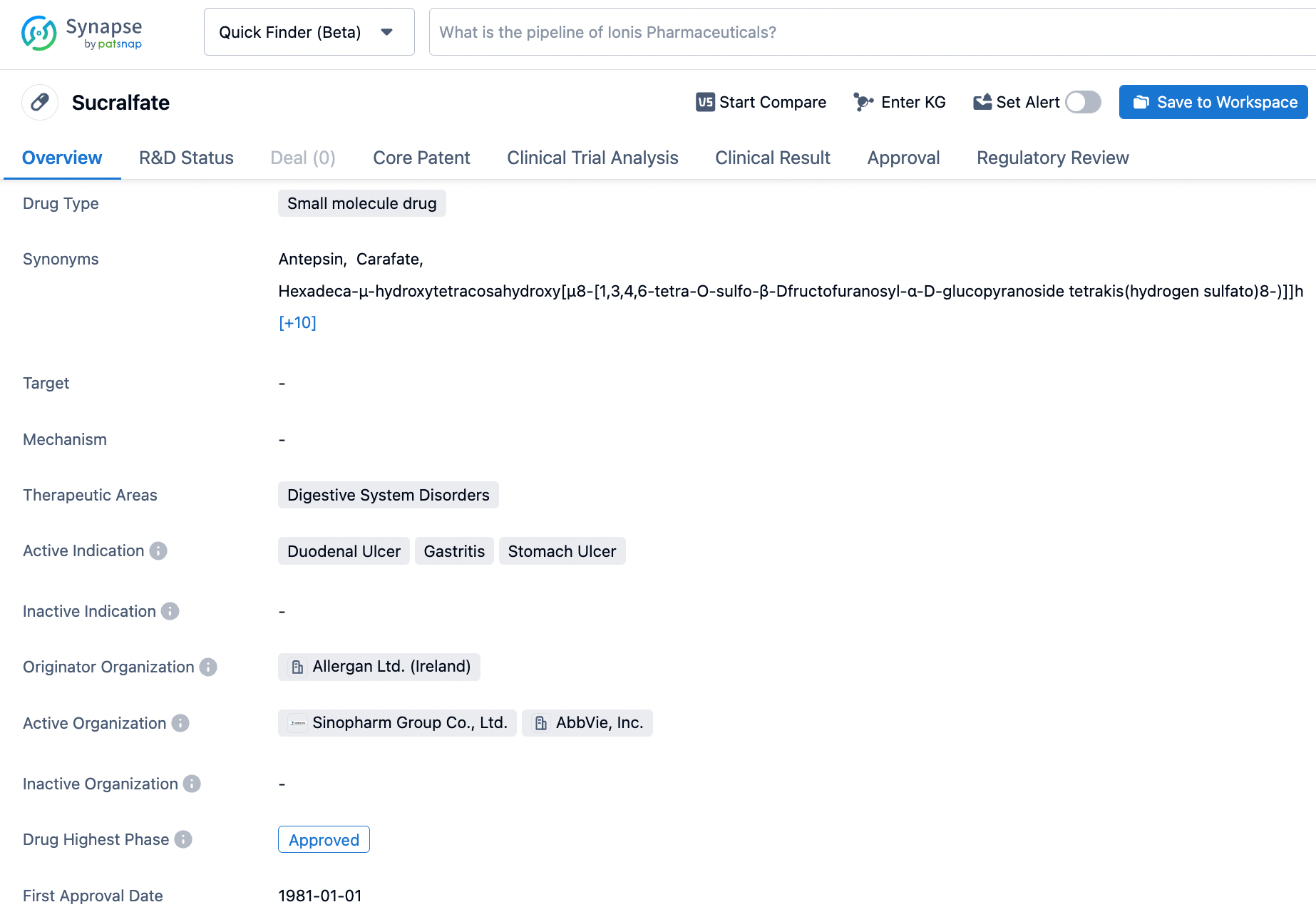
Unlock the Power of Synapse: A Guide to Searching Sucralfate
Sucralfate is a medication that was first approved in Japan in February 1968 by Allergan. The drug is used for the short-term treatment of active duodenal ulcer and works by creating a protective coating over the ulcer site, which helps to promote healing and prevent further damage. Sucralfate's active ingredient is an α-D-glucopyranoside, β-D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. This complex interacts with positively charged proteins in the ulcer site to form a barrier that protects the damaged tissue. Sucralfate is typically taken orally, and should be taken on an empty stomach for maximum effectiveness. While generally well-tolerated, the medication may cause gastrointestinal side effects such as nausea, constipation, and diarrhea. Click on the image below to begin the exploration journey of Sucralfate through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!