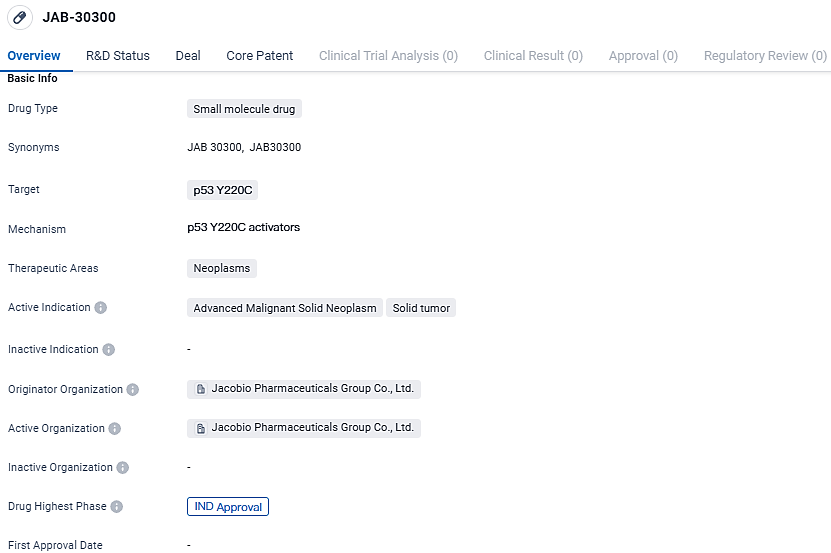
Jacobio Gains U.S. Approval for IND Status of New P53 Y220C Stimulant JAB-30300
Jacobio Pharma, a firm specializing in the development of cancer treatments for previously intractable targets, has recently reported that the United States Food and Drug Administration (FDA) has granted them an Investigational New Drug (IND) authorization for their internally designed compound, JAB-30300, which serves as an activator for the P53 Y220C mutation.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Jacobio is gearing up to launch an early-stage clinical research, Phase I/IIa, targeting complex solid malignancies in the United States, aiming to assess the safety profile and effectiveness of their investigational drug JAB-30300. Concurrently, Jacobio is preparing to file an Investigational New Drug (IND) application in China, with plans to commence clinical trials post-approval.
P53 is widely recognized as the most commonly mutated gene in a variety of cancers, with observed mutations in nearly half of all advanced tumors. JAB-30300, formulated for oral intake, is a small molecule designed to activate P53 in patients who have solid tumors with the specific P53 Y220C mutation.
Research has demonstrated that JAB-30300 possesses an exceptionally high affinity for binding to mutant P53 Y220C proteins. This binding capability has led to tumor shrinkage in various cancer models, including but not limited to, stomach, ovarian, breast, and lung cancers. When used in combination therapy with either chemotherapy agents or inhibitors targeting cancer-promoting proteins, JAB-30300 exhibited enhanced effectiveness, suggesting its potential for combination treatment approaches.
Currently, within the Phase I clinical trial pipeline worldwide, there is only a singular program focusing on P53 Y220C activation. JAB-30300 stands out with the potential to become one of the pioneering P53 Y220C activators to gain market authorization.
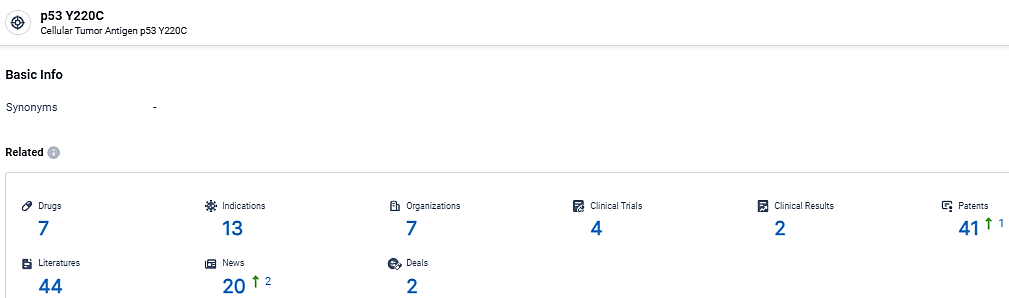
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 5, 2024, there are 7 investigational drugs for the p53 Y220C target, including 13 indications,7 R&D institutions involved, with related clinical trials reaching 4, and as many as 41 patents.
JAB-30300 target the p53 Y220C protein and is primarily intended for the treatment of neoplasms, specifically advanced malignant solid neoplasms and solid tumors. The drug is currently in the highest phase of development, which is IND approval on a global scale. This indicates that JAB-30300 has successfully completed preclinical studies and has been granted permission to proceed with clinical trials in humans.