FDA Approves Kevzara® for Polyarticular Juvenile Idiopathic Arthritis Treatment
Regeneron Pharmaceuticals, Inc. and Sanofi have reported that the U.S. Food and Drug Administration has given approval for Kevzara(sarilumab) to be used in patients who weigh at least 63 kg and suffer from active polyarticular juvenile idiopathic arthritis, a type of arthritis that affects several joints simultaneously.
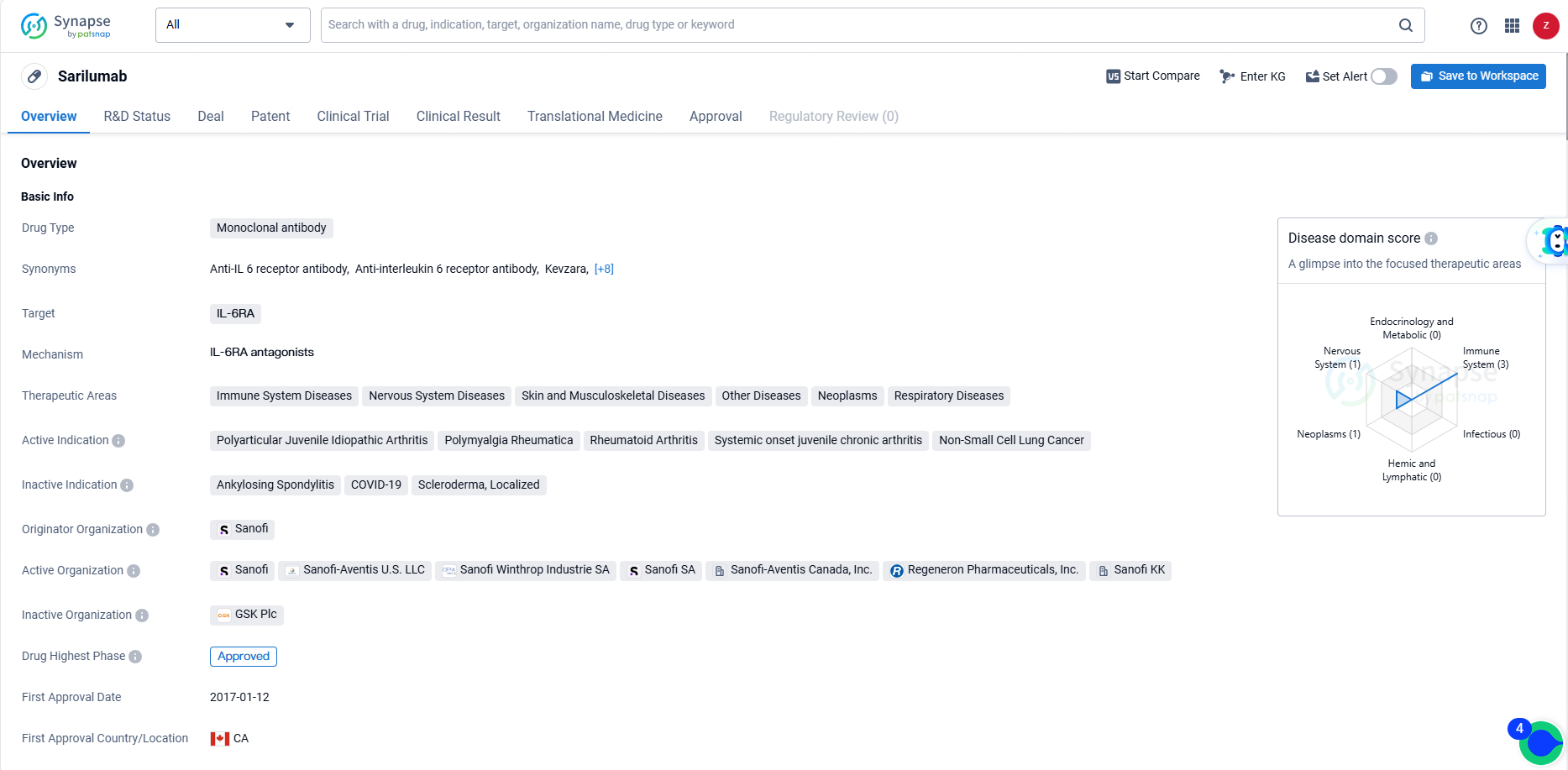
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Polyarticular juvenile idiopathic arthritis can severely affect children, leading to painful inflammation in multiple joints," stated George D. Yancopoulos, M.D., Ph.D., Co-Chairman of the Board, President, and Chief Scientific Officer at Regeneron. "This disease not only disrupts their everyday activities but can also significantly impact their future without proper treatment. The FDA’s approval of Kevzara for polyarticular juvenile idiopathic arthritis offers a new, approved therapeutic option for these vulnerable children and their families to better manage the condition."
The FDA approval for this patient group is backed by robust and well-controlled studies, and pharmacokinetic data derived from adults with rheumatoid arthritis, as well as a dedicated pharmacokinetic, pharmacodynamic, dose-finding, and safety study in pediatric patients with pJIA.
Individuals with pJIA might face joint symptoms including pain, stiffness, and swelling, considerably limiting their everyday activities and making certain tasks exceedingly difficult. Chronic joint inflammation associated with the disease can result in permanent joint damage and impede growth and development.
In the pJIA population, no new adverse reactions or safety concerns were identified compared to those seen in rheumatoid arthritis patients. Common adverse drug reactions for pJIA patients included nasopharyngitis, neutropenia, upper respiratory tract infection, and injection site erythema. Notably, the adverse reaction leading most frequently to permanent discontinuation of Kevzara therapy was neutropenia. Patients treated with Kevzara are generally at an elevated risk of serious infections that could result in hospitalization or death.
"The recent approval of Kevzara introduces a new treatment alternative backed by a proven safety and efficacy profile for pediatric patients with polyarticular juvenile idiopathic arthritis," remarked Brian Foard, Executive Vice President and Head of Specialty Care at Sanofi. "This achievement underscores our continued commitment to providing therapies for younger patients dealing with this chronic and painful joint condition."
Sanofi and Regeneron are dedicated to aiding patients in the U.S. who are prescribed Kevzara in obtaining access to the medication and any necessary support. KevzaraConnect, a comprehensive and specialized program, offers support services to patients throughout the treatment process, assisting eligible patients who are uninsured, underinsured, or in need of copay assistance.
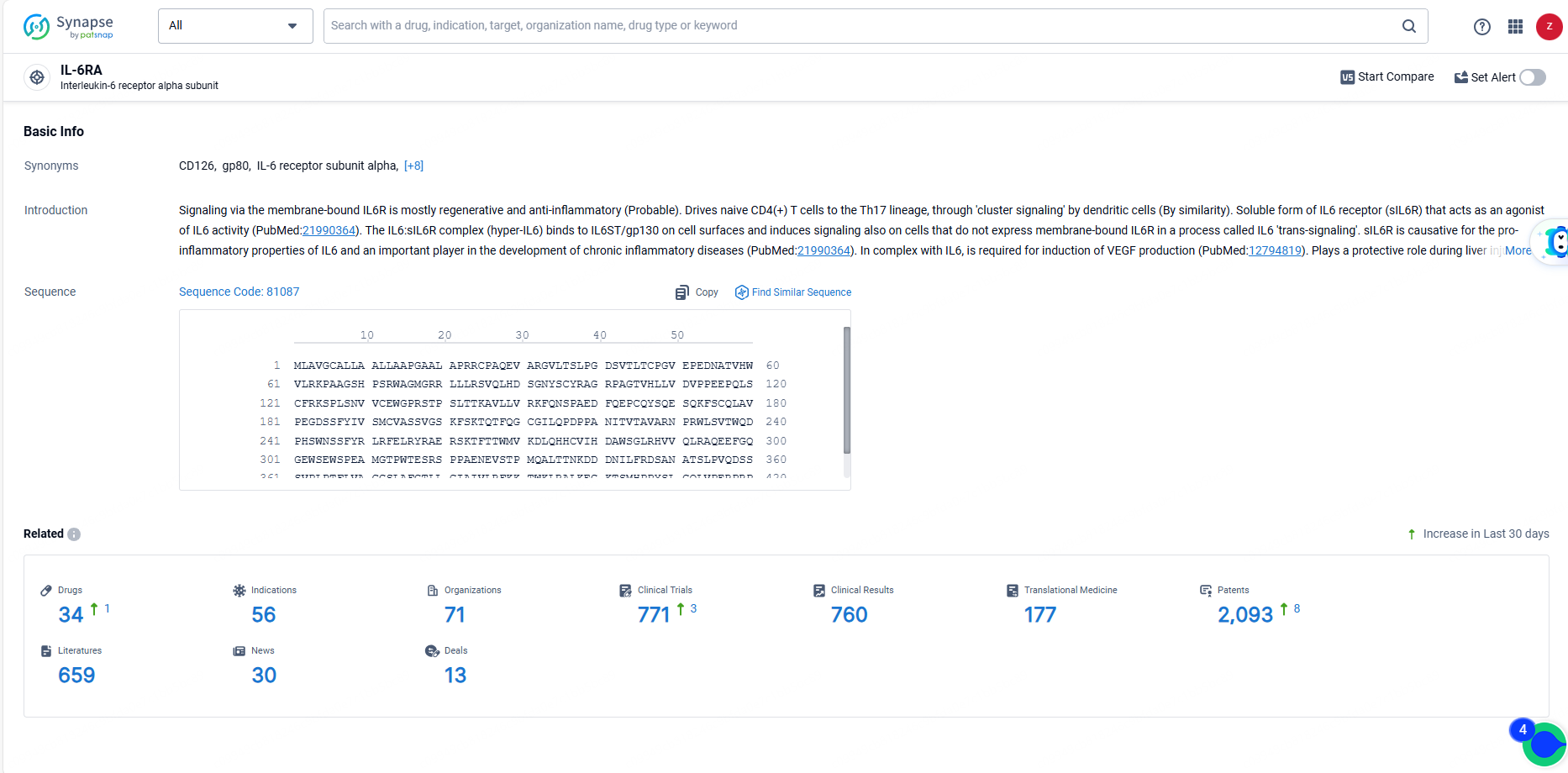
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 14, 2024, there are 34 investigational drugs for the IL-6RA target, including 56 indications, 71 R&D institutions involved, with related clinical trials reaching 771, and as many as 2093 patents.
Sarilumab is a monoclonal antibody drug that targets the IL-6RA and is used in the treatment of various therapeutic areas including immune system diseases, nervous system diseases, skin and musculoskeletal diseases, other diseases, neoplasms, and respiratory diseases. Sarilumab represents a significant advancement in the field of biomedicine and offers new treatment options for patients suffering from the specified diseases. Its approval for use in Non-Small Cell Lung Cancer also highlights its potential in the treatment of cancer, further expanding its impact in the pharmaceutical industry.