Ipsen's Iqirvo® Gains Fast-Track FDA Approval for Primary Biliary Cholangitis Therapy
Ipsen revealed that the U.S. Food and Drug Administration has given accelerated approval for Iqirvo (elafibranor) 80 mg tablets. These tablets are indicated for treating primary biliary cholangitis, either in combination with ursodeoxycholic acid for adults who do not sufficiently respond to UDCA or as a single treatment for those who cannot endure UDCA. Iqirvo is now available for prescription in the U.S. for qualified patients.
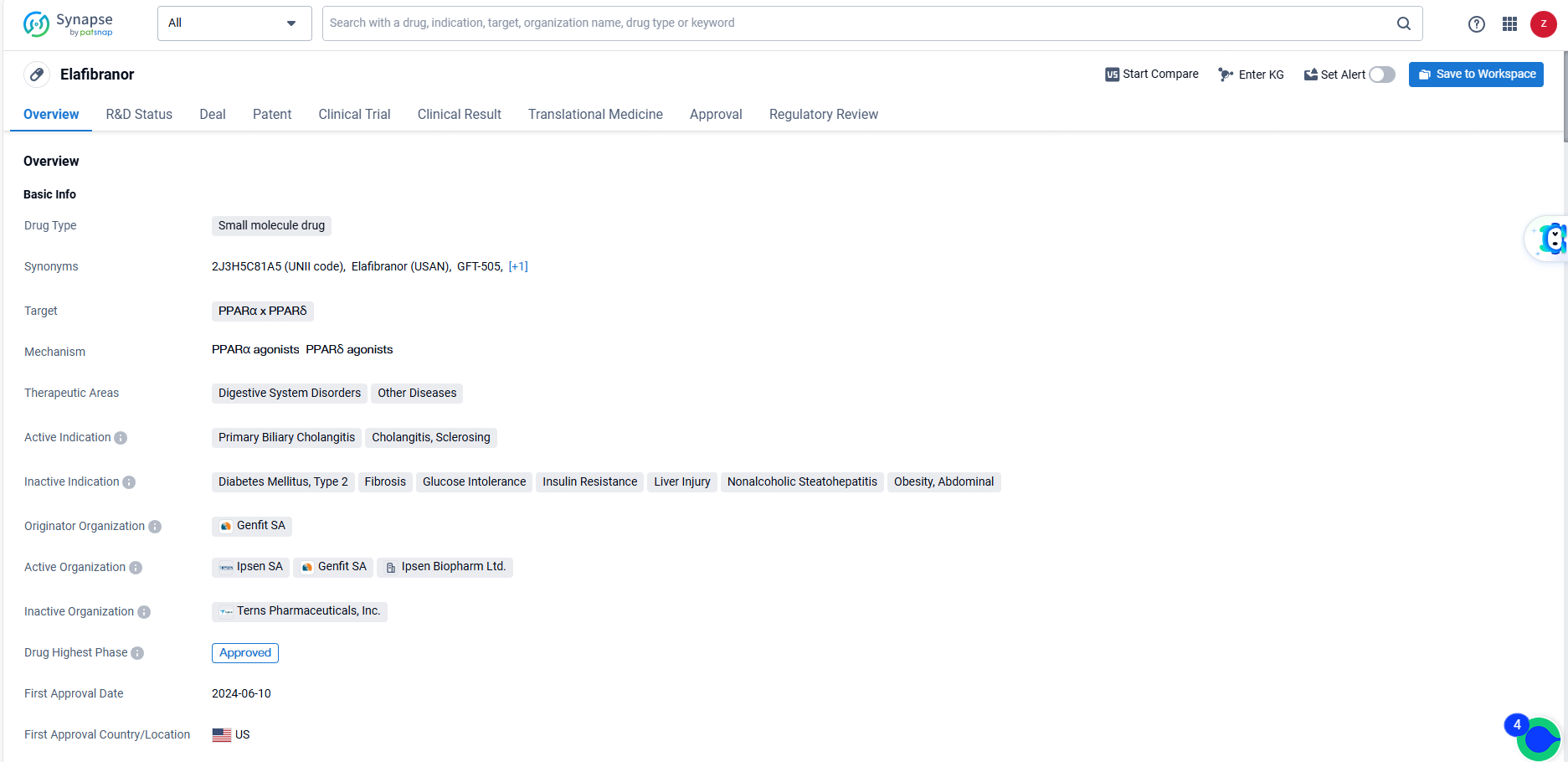
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This indication has received accelerated approval based on a decrease in alkaline phosphatase levels. However, there is no demonstrated evidence of improved survival or prevention of liver decompensation events. Continued approval for this indication might depend on confirming and describing clinical benefits in a follow-up trial. Iqirvo is not recommended for individuals with decompensated cirrhosis or those who develop it.
"A significant number of individuals with PBC find that current treatments fail to control the condition and might even exacerbate symptoms. Without proper management, PBC can progress to liver failure and, in some cases, necessitate a liver transplant," said Christelle Huguet, Executive Vice President and Head of Research and Development at Ipsen.
Iqirvo is a pioneering, once-daily oral peroxisome proliferator-activated receptor (PPAR) agonist. It was licensed from GENFIT in 2021. The accelerated approval of Iqirvo is supported by data from the Phase III ELATIVE trial, which was published in the New England Journal of Medicine1. The ELATIVE trial showed that patients treated with Iqirvo plus UDCA were 13 times more likely to achieve the composite primary endpoint of biochemical response compared to those receiving a placebo plus UDCA.
"Results from the pivotal Phase III ELATIVE clinical trial indicate that Iqirvo is an effective second-line treatment for PBC patients, with a favorable benefit-risk profile," said Dr. Kris Kowdley, Director at Liver Institute Northwest, Washington, and a principal investigator in the ELATIVE study. "The approval of Iqirvo provides U.S. healthcare providers with a new option to meet an unmet need, potentially significantly reducing ALP levels in PBC patients."
Iqirvo has been submitted to both the European Medicines Agency and the UK Medicines and Healthcare products Regulatory Agency for authorization to treat PBC, with final regulatory decisions expected in the second half of 2024.
The FDA's approval of Iqirvo further enhances Ipsen’s portfolio of treatments for rare cholestatic liver diseases available in the U.S. This portfolio also includes our FDA-approved medication for pruritus in patients aged three months and older with progressive familial intrahepatic cholestasis, and for cholestatic pruritus in patients aged 12 months and older with Alagille syndrome.
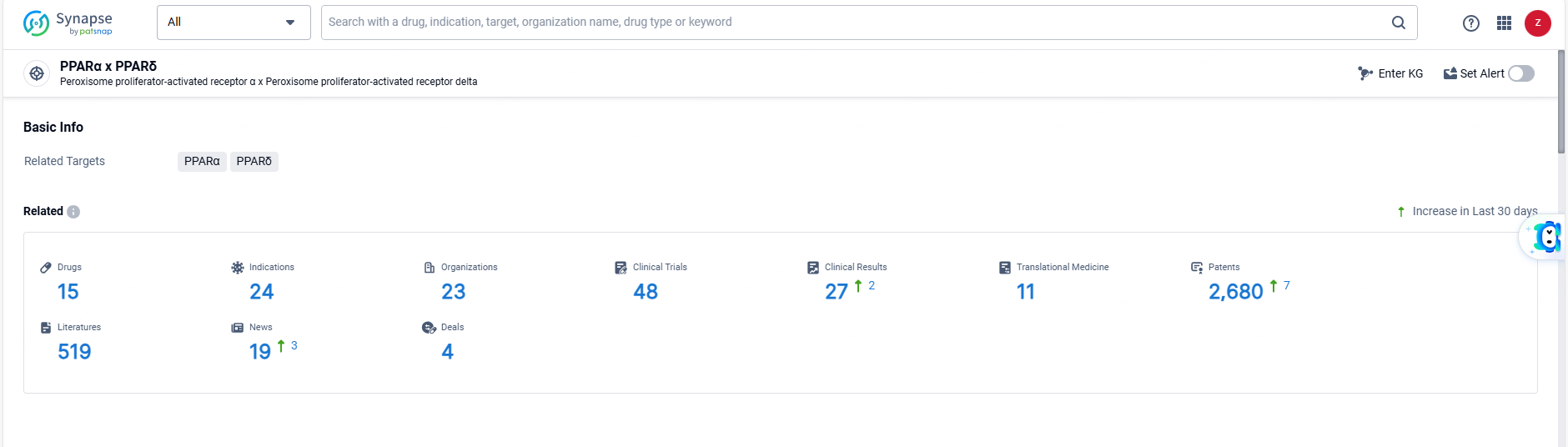
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 14, 2024, there are 15 investigational drugs for the PPARα and PPARδ targets, including 24 indications, 23 R&D institutions involved, with related clinical trials reaching 48, and as many as 2680 patents.
Elafibranor is a small molecule drug with a unique mechanism of action targeting PPARα x PPARδ. Its approval for the treatment of specific digestive system disorders and regulatory designations demonstrate its potential as a valuable therapeutic option. However, further analysis of market dynamics and regulatory challenges in different regions is essential for successful business development strategies.