NMD Pharma Launches Phase 2b Study of NMD670 for Generalized Myasthenia Gravis
NMD Pharma A/S, a biotech firm focused on creating innovative and enhanced therapies for individuals with neuromuscular conditions, announced today that it has administered the first dose to a patient with generalized myasthenia gravis in a Phase 2b clinical trial of NMD670. This follows the FDA's clearance of their IND application in March 2024 to initiate the study. The first patient in the United States was treated under the supervision of Dr. Marc Feinberg at SFM Clinical Research, LLC, located in Boca Raton, Florida, USA.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
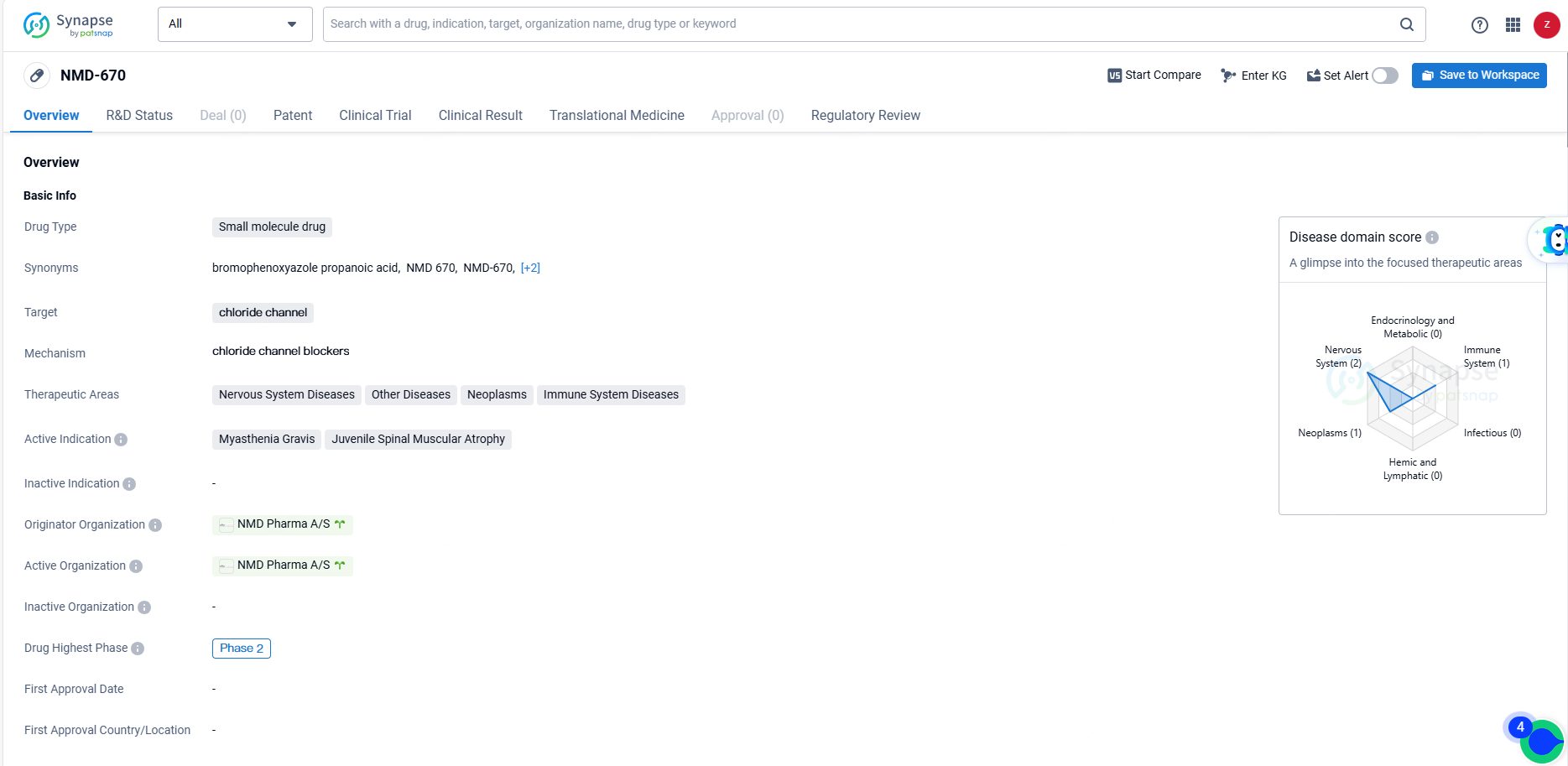
The Phase 2b clinical trial is a double-blinded, placebo-controlled, dose-range-finding study of NMD670, a small molecule oral inhibitor of the skeletal muscle-specific ClC-1 chloride ion channel administered twice daily. This study targets patients with generalized myasthenia gravis (gMG) who test positive for anti-acetylcholine receptor or anti-muscle-specific tyrosine kinase antibodies over a 21-day period. The trial will assess changes in the Quantitative Myasthenia Gravis Total Score and Myasthenia Gravis Activities of Living among other endpoints and will be conducted at clinical sites in both the United States and Europe.
Jorge A. Quiroz, EVP and Chief Medical Officer of NMD Pharma, stated: “Generalized myasthenia gravis is a rare condition marked by dysfunctional neuromuscular transmission, causing severe and fluctuating muscle weakness and fatigue. Despite new and approved therapies targeting the autoimmune response, persistent symptoms remain for many patients with myasthenia gravis. NMD670, which is a novel candidate, is anticipated to enhance muscle strength and endurance, addressing persistent and variable symptoms more effectively.”
Samantha Masterson, President and CEO of the Myasthenia Gravis Foundation of America, remarked: “Many patients with gMG continue to have significant unmet needs. We are hopeful that innovative treatments like NMD670, which focuses on muscle improvement, can offer additional strength and endurance, allowing patients to lead less restricted lives. We are keenly observing the trial’s progression and outcomes, and are hopeful that the promising initial results will translate into substantial clinical benefits for patients.”
NMD670 is a pioneering orally administered small molecule inhibitor of the skeletal muscle-specific ClC-1 chloride ion channel. NMD Pharma has recently reported favorable Phase 1/2a data in Science Translational Medicine, showing the first clinical proof-of-mechanism for CIC-1 inhibitors in gMG patients while also demonstrating the safety and tolerability of a single dose of NMD670 in these patients.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
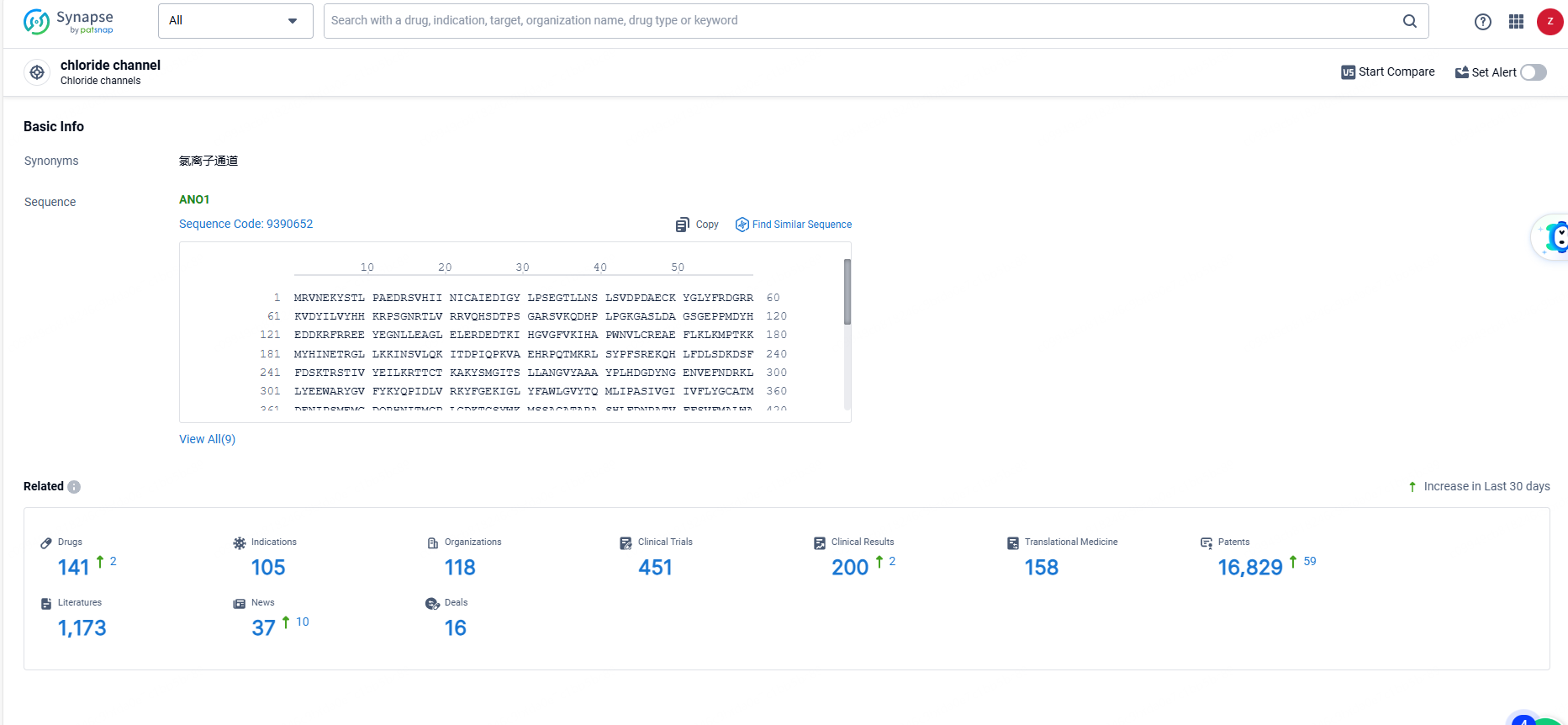
According to the data provided by the Synapse Database, As of June 14, 2024, there are 141 investigational drugs for the chloride ion channel target, including 105 indications, 118 R&D institutions involved, with related clinical trials reaching 451, and as many as 16829 patents.
NMD-670 represents a potential treatment option for a range of diseases, particularly those affecting the nervous system and muscles. Its small molecule nature and specific targeting of chloride channels make it a promising candidate for further development. The Orphan Drug designation also highlights the potential impact of NMD-670 in addressing unmet medical needs for rare diseases. As the drug progresses through clinical trials, further data will be needed to assess its safety and efficacy in treating the specified indications.